, Carlos Capuñay1, Carlos E. Sueldo2 and Juan Mariano Baronio3
(1)
Diagnóstico Maipú, Buenos Aires, Argentina
(2)
University of California, San Francisco, CA, USA
(3)
CEGYR, Buenos Aires, Argentina
Causes of infertility by uterine factors have a prevalence of a 10 %.
The uterus participates in key processes of the reproductive system that involve the transport of spermatozoids, embryo implantation and fetal nutrition. It is for this reason that congenital uterine anomalies (unicornuate, bicornuate, septate uterus, etc.) and acquired pathologies (endometrial polyps, intrauterine synechiae and fibroids) can negatively influence fertility [1–7].
Uterine pathology is classified in intraluminal pathology and mural pathology. The intraluminal pathologies include alterations that may be found in the interior of the uterine cavity. In the present chapter these topics are focused on exclusively intraluminal findings.
There are various diagnostic methods for the evaluation of the uterine cavity such as conventional hysterosalpingography (HSG) [8–11], sonohysterography [12–14], magnetic resonance imaging [15, 16] and hysteroscopy [17, 18]. The first three are exclusively diagnostic modalities while the hysteroscopy is a diagnostic and therapeutic study.
HSG is the method most commonly used in the study of infertile patients because it is easy to perform and has low costs. Nevertheless, this modality is relatively invasive because it requires clamping of the uterine neck in order to place a cannula in the external cervical os to instill contrast into the uterine cavity [19, 20]. HSG also requires traction of the cervical neck to avoid the overlapping of structures of the gynecologic apparatus that may impede the visualization and detection of the pathology. The invasiveness of this procedure can be associated with complications such as bleeding, infections and vasovagal reactions due to the intra-study discomfort.
Sonohysterography is a less invasive exam compared to the HSG which permits the adequate evaluation of the uterine cavity via the instillation of physiologic solution [21]. However, this imaging technique is not widely used because it does not provide information on the cervix or of the fallopian tubes. So other diagnostic methods are necessary to complement this information.
Magnetic resonance imaging is a study that is carried out principally for the diagnosis of uterine malformations [15, 22, 23]. However, it does not allow detecting all associated intra-luminal pathology and does not offer information of the fallopian tubes.
Hysteroscopy is a diagnostic and therapeutic method and is considered the gold standard modality for the evaluation of the uterus, with the disadvantage that it requires anesthesia [24]. This technique is most commonly utilized to confirm suspicious findings in other diagnostic methods [25].
Currently, a new non invasive diagnostic tool is available, the virtual hysterosalpingography (VHSG) [2, 5]. This new modality provides, in few seconds and with a very low radiation dose, excellent information on the uterus and offers, simultaneously, the evaluation of the cervix, the fallopian tubes and the associated intrapelvic pathology.
Within the intra-luminal pathology there exist diverse alterations that may be found in the interior of the uterus:
Synechiae
Endometrial infections sequelae.
Other endometrial processes: hypoplasia, endometrial polyps, adenomyosis.
Neoplasias: submucous myomas, endometrial carcinoma.
Intrauterine devices.
Post surgery changes.
Intrauterine Synechiae
They are fibrous bands of conjunctive tissue that stick to the uterine walls between themselves. Their prevalence in the general population is of 1.5 % and this percentage increases to 13 % if a history of infertility and repeated abortions exists [26–28]. Intrauterine synechiae represent a scar in the uterine cavity and are the result of an infection or trauma, frequently after a birth or post-abortion curettage. Trauma on the uterine wall induces the scarring and causes fibrosis. Fibrosis can be minor and involve a small section of the uterine wall or be extensive with a diffuse commitment and obliteration of various sectors of the cavity.
The clinical manifestations are commonly menstrual problems like amenorrhea, oligomenorrhea, repeated abortions, infertility or premature births.
The American Society of Fertility and Reproductive Medicine classifies the synechiae according to observed findings in radiologic and hysteroscopy studies, and in menstrual changes determining their severity.
Patients present themselves with menstrual dysfunction, infertility, repeated abortions or post-pregnancy complications. The association of infertility and synechiae is known as the Asherman Syndrome [29].
Synechiae can be located anywhere in the uterus. When they involve the cervical region they produce, in certain cases, stenosis of the internal cervical orifice. They can also generate stenosis of the tubarian ostium.
HSG : the uterine synechiae are observed as filling defects which distort the morphology of the uterine cavity. They acquire an irregular and angular shape and do not present mobility (Fig. 6.1). They are easily differentiated from endometrial polyps because these have regular edges [37] (Fig. 6.2).
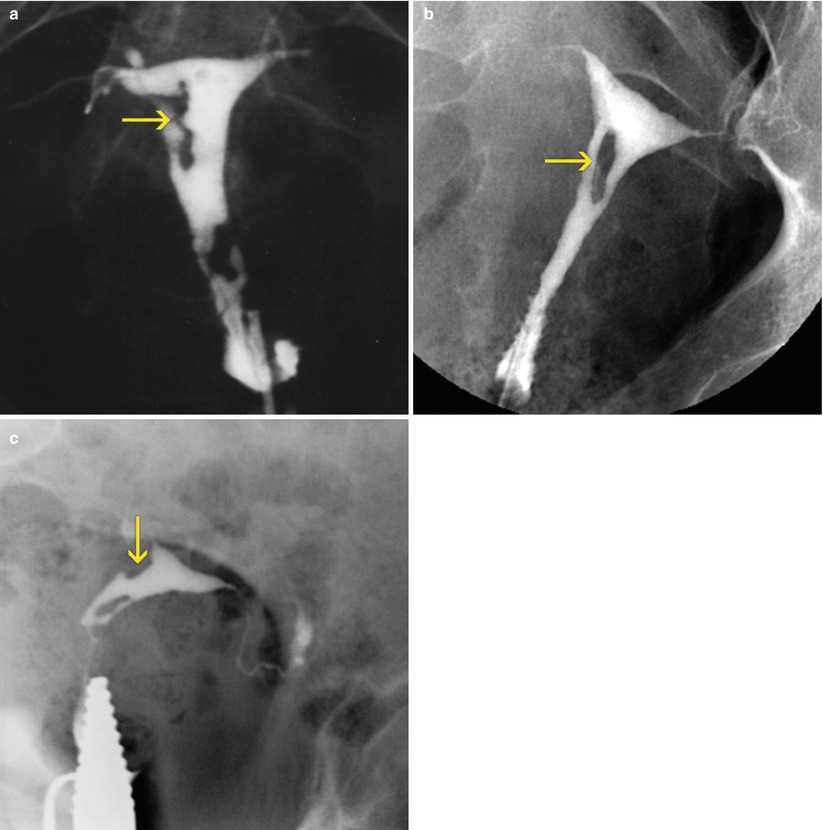
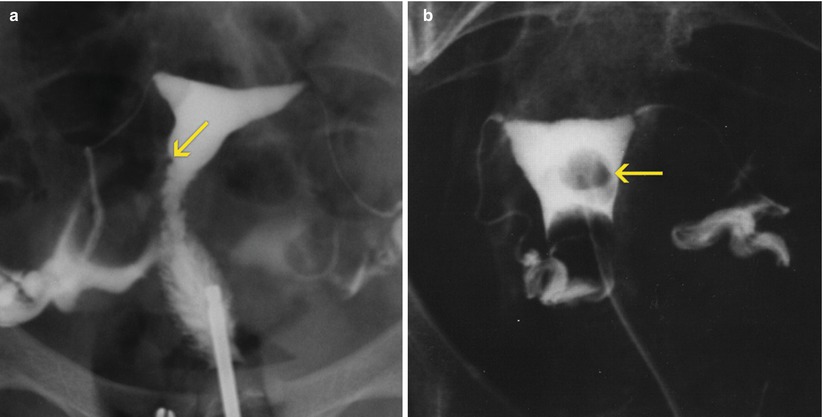

Fig. 6.1
(a–c) Uterine synechiae seen on HSG images. Multiple filling defects are observed in the uterine cavity with irregular edges (arrows) compatible with synechiae

Fig. 6.2
Uterine polyps seen on HSG images. (a) Small polyp on the right lateral wall of the uterine silhouette (arrow). (b) Large polyp in the medial aspect of the uterine cavity (arrow)
To make a correct diagnosis, the progressive filling of the uterine cavity is essential.
VHSG: this modality identifies the uterine synechiae in the different postprocessing techniques [38, 39].
Multiplanar reconstructions show irregular elevated lesions with soft tissue density which extend from the uterine walls. They can also be located in the isthmus-cervical region, center or fundus of the uterine cavity or adjacent to cornual regions (Figs. 6.3, 6.4, and 6.5). If they comprise various regions simultaneously, findings can be diffuse. (Figs. 6.6 and 6.7).
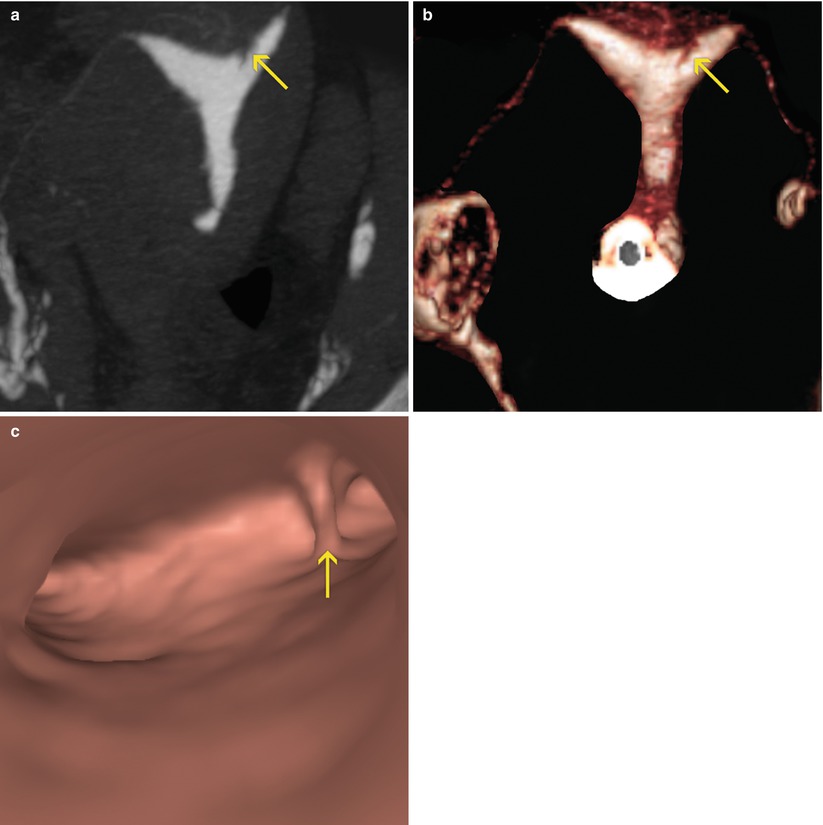
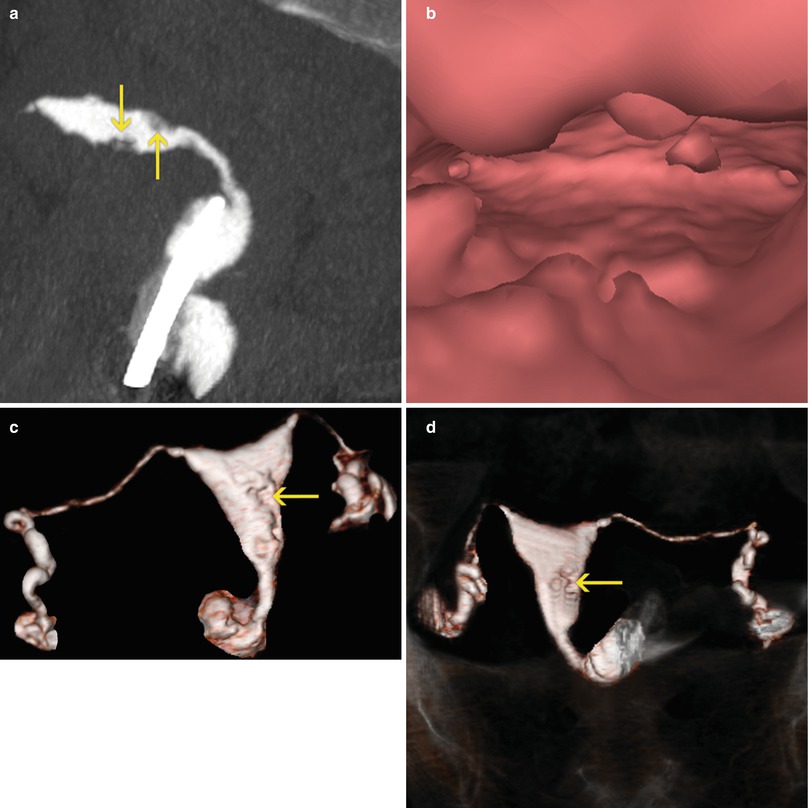
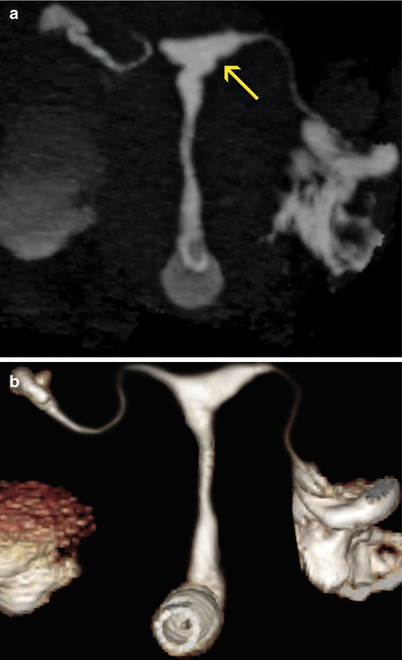
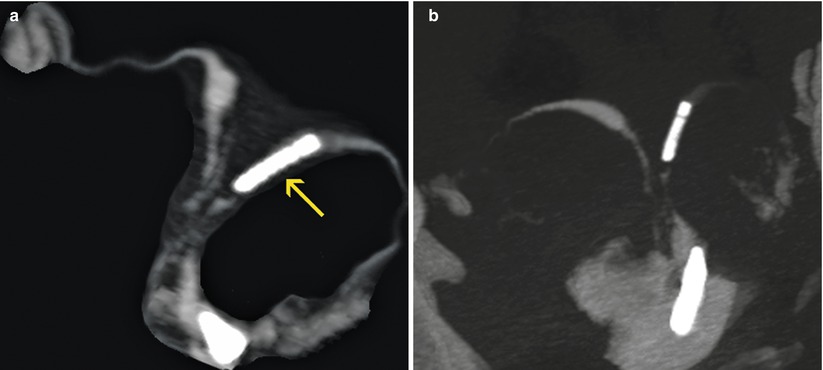
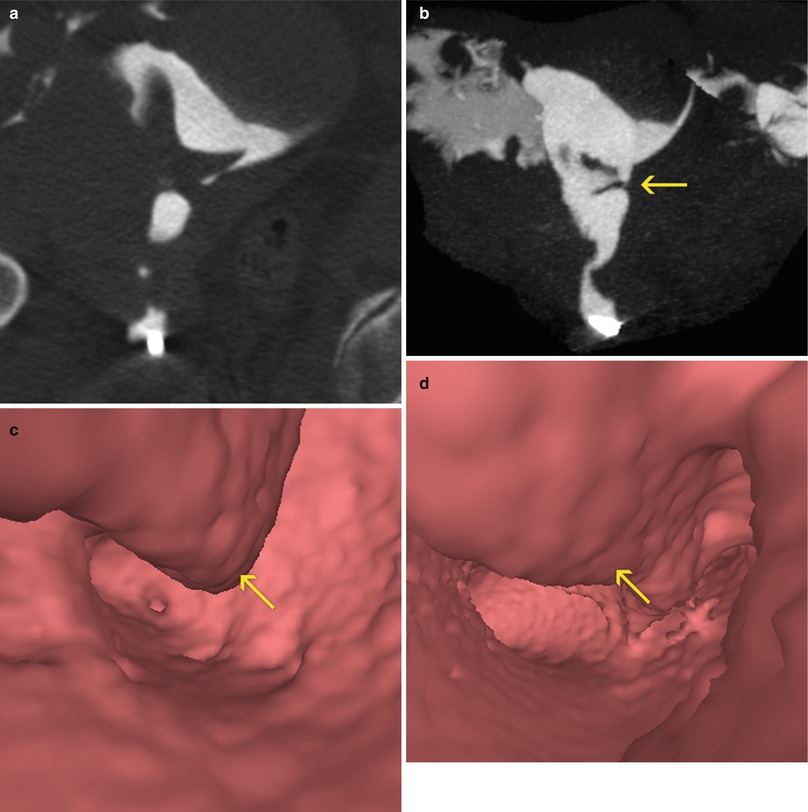

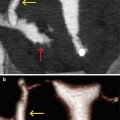
Fig. 6.3
Synechiae in uterine fundus adjacent to left horn. (a) Coronal maximum intensity projection image which shows a lineal image (arrow) at the level of the uterine fundus compatible with synechiae. (b) 3D volume rendering image which illustrates similar findings (arrow). (c) Virtual endoscopy image of the uterine fundus which exhibits a lineal band that joins the anterior and posterior walls of the uterus (synechiae) (arrow)

Fig. 6.4
Uterine synechiae. (a) Sagittal maximum intensity projection image that shows an anteverted uterus, which presents multiple filling defects compatible with synechiae (arrows). (b) Virtual endoscopy image which illustrates endoluminal lesions. (c, d) 3D volume rendering images which exhibit irregularities on the wall corresponding to synechiae (arrows)

Fig. 6.5
Uterine synechiae. (a) Coronal maximum intensity projection image which shows a uterus with irregular edges (arrow) due to the presence of synechiae. (b) 3D volume rendering image which illustrates similar findings

Fig. 6.6
Diffuse uterine synechiae. (a, b) Maximum intensity projection images that show a bicornuate uterus with intrauterine contraceptive device in the left horn (arrow). Multiple intraluminal filling defects are observed in the corpus and proximal sector of both uterine horns compatible with diffuse synechiae

Fig. 6.7
Diffuse uterine synechiae. (a) Coronal multiplanar reconstruction image which shows a uterus multiple filling defects in the proximal and medial aspects of the uterine cavity compatible with synechiae. (b) Coronal maximum intensity projection image which exhibits an irregular uterus with intraluminal filling defects (arrow). (c, d) Virtual endoscopy images that illustrate a uterus with irregular walls due to diffuse synechiae (arrows)
Maximum intensity projection images (MIP) may exhibit sections of irregularities on the uterine wall, although they constitute the reconstruction format which offers reduced information in the evaluation of this pathology (Fig. 6.8).
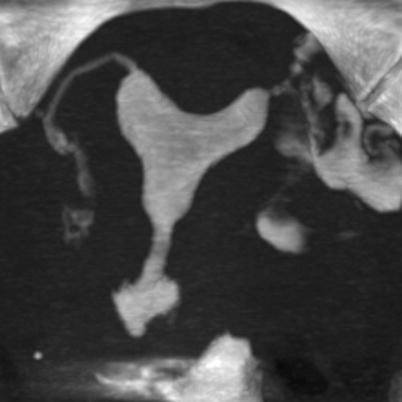

Fig. 6.8
Maximum intensity projection (MIP) images for the evaluation of synechiae. The MIP images possess limitations in the identification of intraluminal pathologies such as synechiae and polyps. Its main utility is for the evaluation of the Fallopian tubes
Volume rendering reconstructions show filling defect where the synechiae are located (Fig. 6.9).
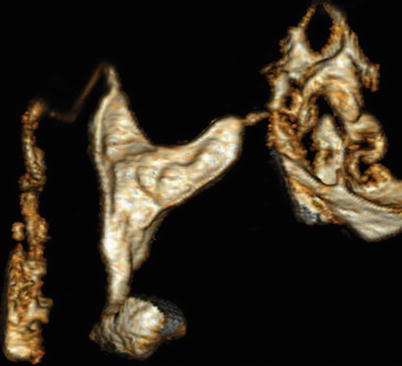

Fig. 6.9
3D volume rendering images for the evaluation of synechiae. These 3D images are ideal in the evaluation of this pathology, showing filling defect areas. The appearance of the uterine morphology contributes to a precise diagnosis
Virtual endoscopy images expose endoluminal lesions that project towards the uterine lumen reducing its size. The level of compromise depends on the extension of the synechiae (Fig. 6.10).
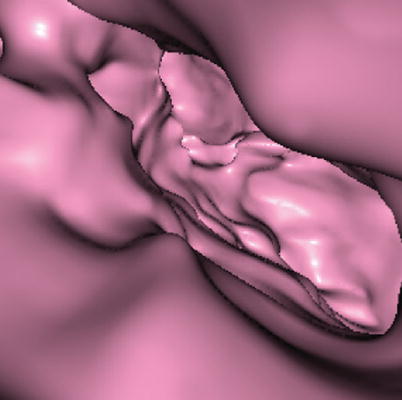

Fig. 6.10
Virtual endoscopy images for the evaluation of synechiae. These images offer intraluminal views which provide the location, extension and compromise of synechiae
Hysteroscopy: is a diagnostic and therapeutic method at the same time because it permits the extraction of the adhesions once confirmed. A Foley catheter is introduced which is placed in the uterine cavity and a balloon is insufflated to keep the walls separated.
The synechiae are extracted and, later, antibiotics are administered post-operatively for a week. In the post-surgery, estrogen is supplied twice a day during 30 days and then medroxyprogesterone for 7 consecutive days. In general, after a month of treatment, an improvement of the symptoms and uterine morphology is observed, although the rate of infertility is not significantly modified [40, 41].
Endometrial Infections
Endometrial infections can be acute or chronic. The acute infections are in general of bacterium etiology, being Chlamydia trachomatis and Ureaplasma urealyticum the most frequent agents. The most common causes of the chronic infections are due to intrauterine devices, tuberculosis or gonococcia [42–44].
Frequently, origin is the post-delivery infection, due to instrumentation in the uterine cavity or because of the propagation of adjacent infections of the genital tract.
Acute infections can progress to chronic and end up generating extensive uterine synechiae with severe cavity deformation (Fig. 6.11)
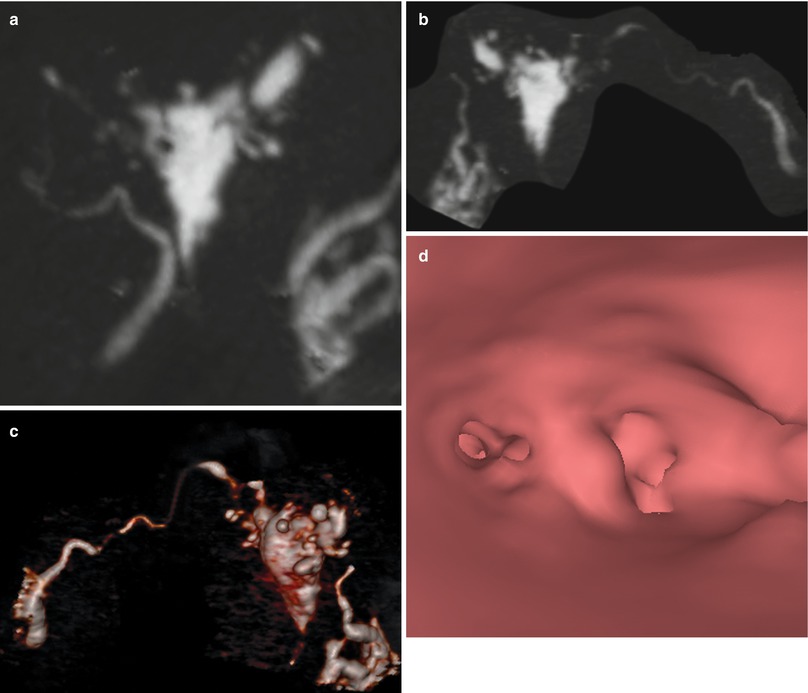

Fig. 6.11
(a, b) Maximum intensity projection images showing a totally deformed uterus with irregular edges due to extensive synechiae. (c) 3D volume rendering image illustrating similar findings. (d) Virtual endoscopy image showing a small uterine cavity due to extensive synechiae
Other Endometrial Processes
Hypoplasia
Hyperplasia
Endometrial polyps
Adenomyosis
Endometrial Hypoplasia
This pathology is frequently observed in old women with premature menopause. It can also be seen in young patients due to an ovary dysfunction or because of the use of oral contraceptives.
Atrophic endometrium produces a diffuse irregularity in the uterine walls.
Available methods for its diagnosis are HSG and VHSG.
HSG: shows uterus with diffuse parietal irregularities and spiculated aspect [45, 46] (Fig. 6.12).
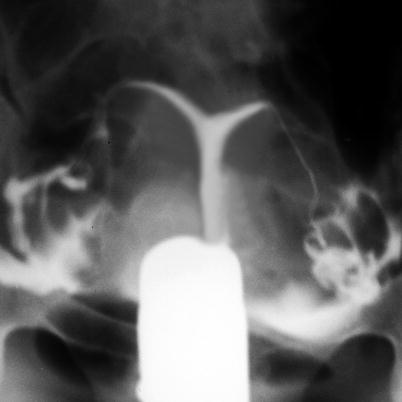

Fig. 6.12
Hypoplasia of the uterus seen on HSG exam
VHSG : shows diverse forms of two-dimensional and tridimensional reconstructions show a uterus with diffuse irregular walls [38, 39] (Fig. 6.13). These irregularities can also be identified endoluminally at the level of all of the walls.
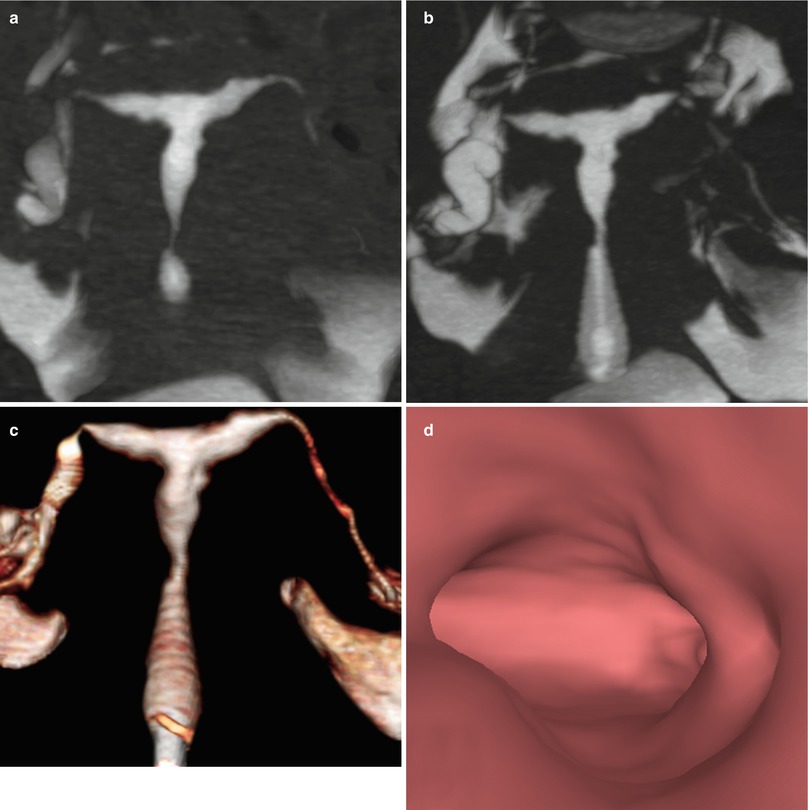

Fig. 6.13
Hypoplasia of the uterus seen on VHSG exam. (a, b) Maximum intensity projection images showing a small uterus of irregular edges. (c) 3D volume rendering image illustrating similar findings. (d) Virtual endoscopy image showing irregular endoluminal edges
Hyperlapsia
Constituted by a proliferation of the glands. The patients may have metrorrhagia.
Useful diagnostic methods are pelvic and transvaginal ultrasound, VSHG and hysteroscopy [47].
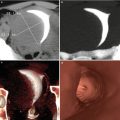
Ultrasound: shows a bigger and wider endometrium of 10 mm (Fig. 6.14). Ovary tumors can be observed.
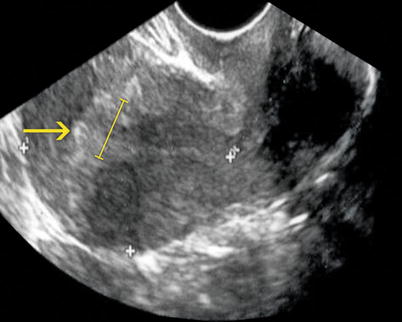
Fig. 6.14
Endometrial hyperplasia seen on transvaginal ultrasound. Longitudinal image of the uterus showing an abnormally thickened endometrium of 14 mm (arrow)
VHSG: shows an irregular endometrium that can be appreciated in multiplanar reconstructions, volume rendering and virtual endoscopy images.
Multiplanar reconstructions: irregular margins of the lumen can be observed. They show an endometrium with diffuse multiple elevated pseudopolyps that project into the lumen with no preponderant lesion (Fig. 6.15).
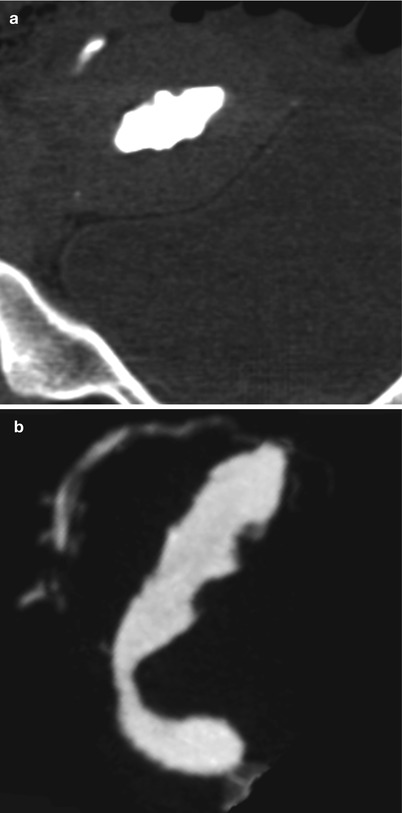
Fig. 6.15
CT multiplanar reconstruction (MPR) image of endometrial hyperplasia. (a) Coronal MPR image with soft tissue window. (b) Sagittal MPR image with soft tissue window showing an irregular uterine cavity
Volume rendering: exhibits irregular uterine margins, frequently associated with a reduced cavity due to endometrial hypertrophy (Fig. 6.16).
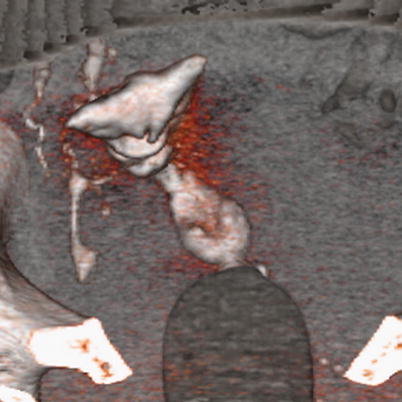
Fig. 6.16
3D volume rendering image of endometrial hyperplasia. Uterus with irregular edges and walls due to an abnormally thickened endometrium
Virtual endoscopy: shows a small uterine cavity with irregular hypertrophic mucosal folds with polypoid appearance (Fig. 6.17).
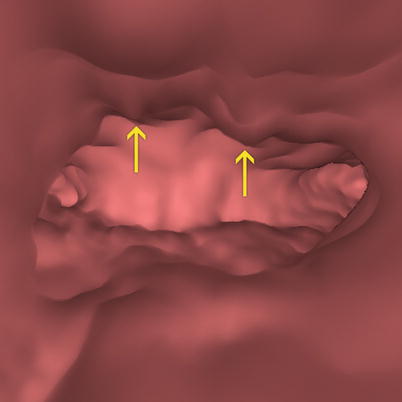
Fig. 6.17
Virtual endoscopy image of an abnormally thickened endometrium. An irregular cavity due to hypertrophic mucosal folds with polypoid appearance is observed (arrows)
Endometrial Polyps
They constitute focal overgrowths of tissue in the endometrium. They contain glands, fibrous stroma and vessels. They occur more frequently in the fifth decade. Their growth is sensitive to the hormone levels. It is estimated that they affect 11–24 % of infertile women [48].
Risk factors include age, arterial hypertension, diabetes and hormonal tamoxifen treatment among others.
Polyps can be asymptomatic or be associated to uterine bleeding. They are benign lesions and rarely become malignant. Only 0.5 % of cases correspond to an endometrial carcinoma [49–51].
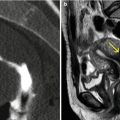
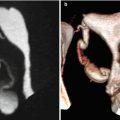
Morphologically they are sessile (Fig. 6.18) or pedunculated (Fig. 6.19). Pedunculated polyps can even pass through the internal cervical orifice (Fig. 6.24).
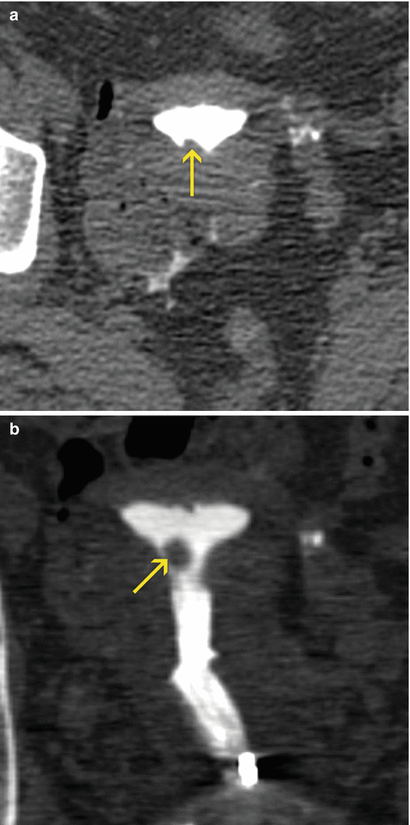
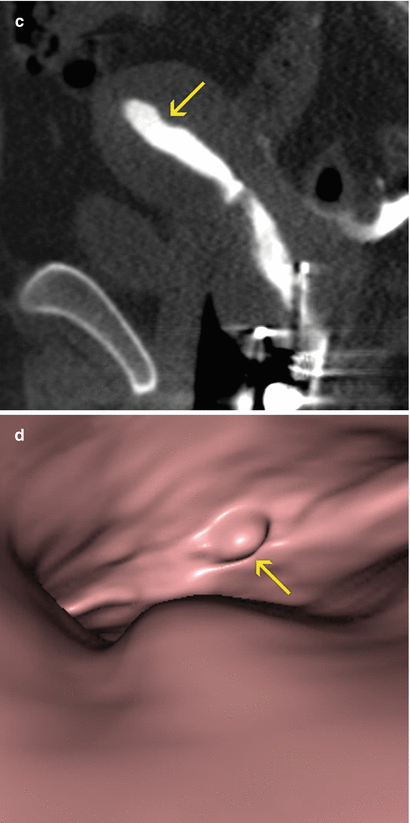
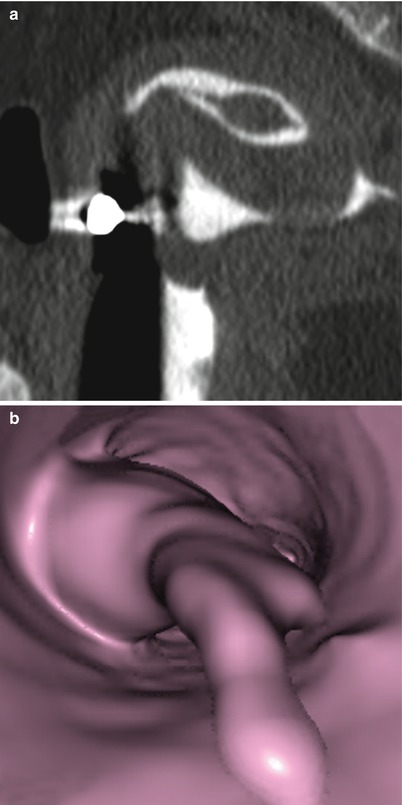


Fig. 6.18
Endometrial sessile polyp. (a) Axial CT image with soft tissue window which shows an elevated lesion in the anterior wall of the uterine cavity compatible with a sessile polyp (arrow). (b, c) Coronal and sagittal multiplanar reconstruction images with soft tissue window that illustrate a polyp lesion at the level of the uterine cavity (arrow). (d) Virtual endoscopy image which exhibits the polyp (arrow)

Fig. 6.19
Endometrial pedunculated polyp. (a) Sagittal multiplanar reconstruction image with soft tissue window which shows a pedunculated polyp. The head of the polyp projects towards the uterine fundus. (b) Virtual endoscopy images which shows the polyp from the cervical region
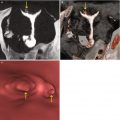
They can be solitary (Fig. 6.20) or in groups (Fig. 6.21). In 75 % of cases they are alone.
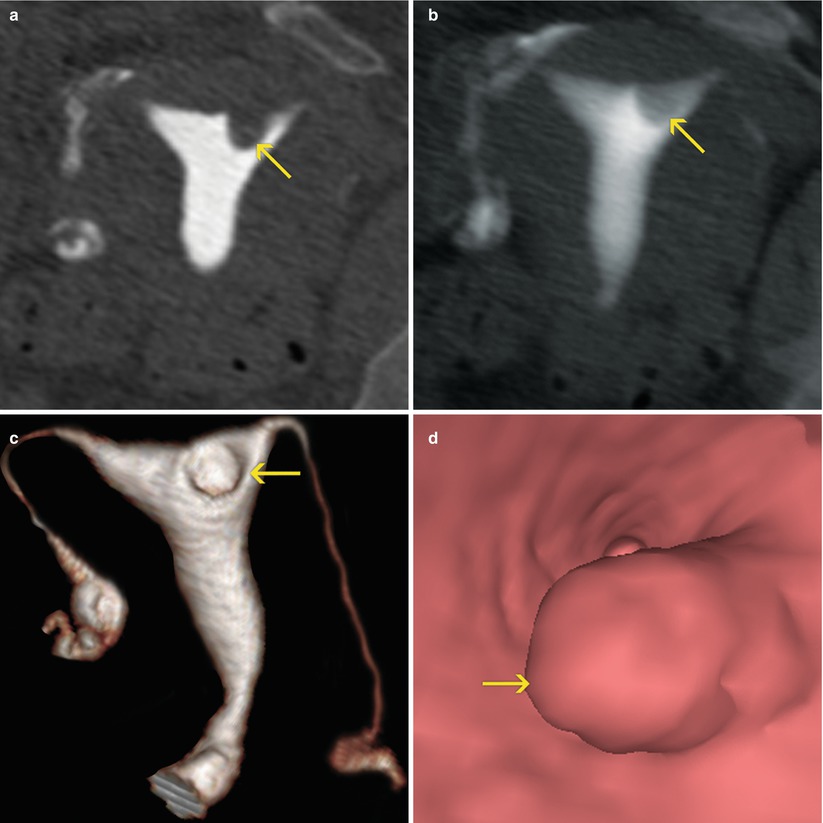
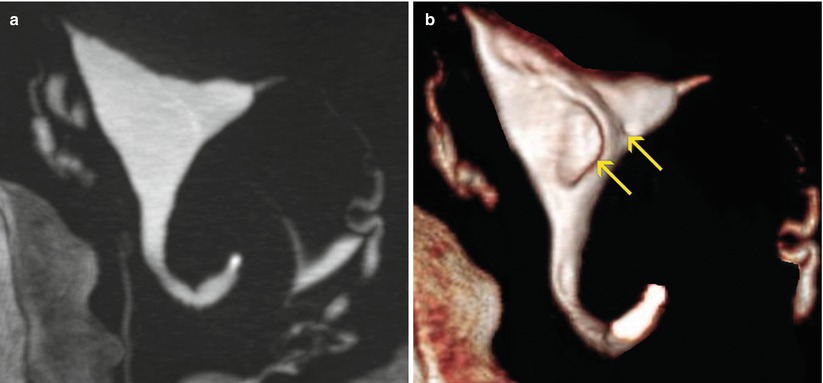

Fig. 6.20
Isolated endometrial polyp (arrows). (a) Coronal multiplanar reconstruction (MPR) image with soft tissue window which shows an isolated elevated lesion at the level of the uterine fundus adjacent to the left horn compatible with polyp. (b) 10-mm coronal MPR image with soft tissue window illustrates similar findings. (c) 3D volume rendering image that illustrates similar findings. (d) Virtual endoscopy image. The endoluminal elevated lesion can be clearly observed

Fig. 6.21
Multiple endometrial polyps. (a) Maximum intensity projection image which shows a normal uterine cavity with no evidence of further findings. This type of image reconstruction does not correctly visualize the endoluminal lesions. (b) 3D volume rendering image which illustrates the presence of two elevated lesion: a small one and a large one (arrows) near the right uterine horn
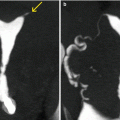
Their size is variable, from scarce millimeters (Fig. 6.22) to a couple of centimeters (Fig. 6.23).
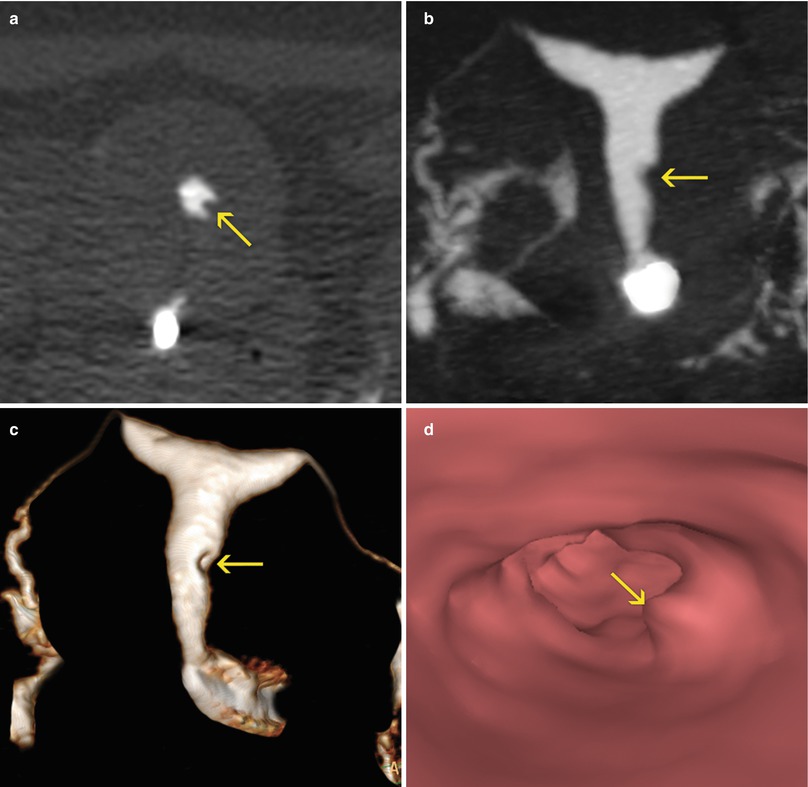

Fig. 6.22




Millimetric endometrial polyp. (a) Axial CT image with soft tissue window which shows an elevated lesion in the left lateral wall of the uterus (arrow) compatible with a polyp. (b, c) Coronal maximum intensity projection and 3D volume rendering images showing a small filling defect in the left lateral wall (arrow). (d) Virtual endoscopy image which shows the polyp (arrow)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree