Patient Safety in Cardiovascular Magnetic Resonance Imaging
Mark Doyle
Safety, as with any diagnostic medical technique, is a critical issue, and safety procedures and rules should, at a minimum, be familiar to the physicians responsible for the scan and to the technician performing the scan. Major sources of safety concern are as follows:
The potential of the magnetic field to move or dislodge an object or device
The possibility that the radiofrequency (RF) field will heat a conductor such as a pacemaker electrode
The potential for inducing electric currents in a conductor, again such as a pacing electrode
The misinterpretation of artifacts that can confound diagnoses
PACEMAKERS
The most common implanted devices that generally excludes the patient from having a magnetic resonance imaging (MRI) study are:
Pacemakers
○ Permanent
○ Temporary
Automated implantable cardiovascular defibrillators
At least six deaths have occurred in patients with pacemakers, thought to be related to an MRI procedure
However, exclusion of patients with pacemakers is not absolutely prohibited, and a careful risk-benefit analysis should be performed before allowing a patient with a pacemaker into the scanner. First, the patient should not be pacemaker dependant, the pacemaker should be put into the synchronous mode, and sequences with low specific absorption rate (SAR) should be used. As a precaution, allow extra time between individual scans to permit heat that might be generated to dissipate.
PROSTHETIC HEART VALVES
Prosthetic heart valves are generally magnetic resonance (MR) compatible. A possible exception is the pre-6,000 series Starr-Edwards valve (a caged ball device used clinically several decades ago). To our knowledge there has never been a report of an untoward incident involving a heart valve prosthesis.
NONHAZARDOUS IMPLANTS
Nonhazardous implants include the following (when designated as MR compatible):
Bypass graft clips
Properly installed intravascular coils
Stents
However, all of these may result in an MR signal artifact.
HAZARDOUS IMPLANTS
Hazardous implants include the following:
Intracranial aneurysm clips
Ocular prostheses
Cochlear prostheses
Penile prostheses
EXTRANEOUS METAL DEVICES AND OBJECTS
Technicians, especially, must also be aware of the potential danger associated with metallic devices that may accompany the patient, including the following:
Wheelchairs
IV poles
Oxygen tanks
Iron shot filled hemostatic “sandbags”
Pens
Beepers
Cell phones
Hairpins (bobby pins)
Shoes
Badge tags
These, and other devices, may become dangerous projectiles in the vicinity of an MR system (see Fig. 37-1). The most dangerous time is when the patient is in the scanner bore and another person is in the room. It is imperative that all extraneous objects (that could contain metal) be removed from personnel entering the scanner room. It is important to note that just because something is marked “MR compatible” it does not mean that it truly is.
It may have been refurbished with noncompatible parts.
It may have been mislabeled.
It may be attached to something which is not MR safe.
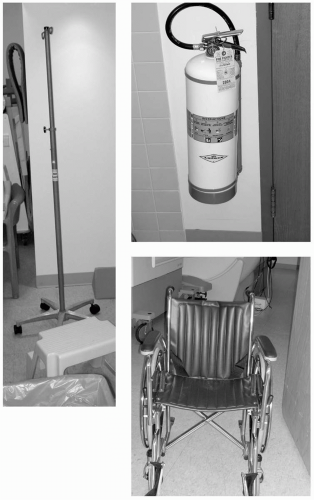 FIGURE 37-1 Extraneous metal objects that, at some time, may find their way into the scanner room should be magnetic resonance imaging (MRI) compatible and clearly labeled as such. |
 FIGURE 37-2 Power injectors are required to administer contrast agent and saline flush, and should be of a special design to make them magnetic resonance (MR) safe. As always, a safety label should be prominently placed on all apparatus allowed in the scanner room. |
It is advised to test everything that claims to be MR safe yourself when no one is in the magnet, and beware of changes and updates made to these objects (see Fig. 37-2).
RESOURCE
A useful resource is the web site that lists current devices and their degree of MR compatibility:
http://www.MRIsafety.com/
The list is constantly being updated.
STATIC MAGNETIC FIELD ZONES
It has been proposed that the following three zones be established around the scanner:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree