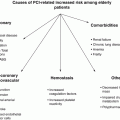
Characteristics and comorbidities of women with CVD
Older age
Hypertension
Hyperlipidemia
Diabetes
Later presentation
Cardiogenic shock
Renal insufficiency
Comorbidities
Women have a higher incidence of comorbidities than men at the time of presentation for acute coronary syndromes (ACS), such as hypertension, hyperlipidemia, and diabetes [11]. Women with ACS also tend to present later with dynamic EKG changes, more disabling ischemic symptoms, and even cardiogenic shock, often despite no prior history of MI, PCI, or coronary artery bypass graft surgery [11, 12]. Women are more likely to have renal insufficiency which may have a significant prognostic impact, especially post-STEMI [13]. On the other hand, women are less likely to have a history of smoking, prior MI, and coronary revascularization compared to men [9, 11].
Atypical Presentation
Patient presentation can greatly influence the timeliness of diagnosis and treatment of ACS and MI. Differences in the presenting symptoms between women and men with ST-segment elevation myocardial infarction (STEMI) have been thought to contribute to the rate of 30-day mortality among women [13]. The most common symptom of ACS for both men and women is chest pain (94 % vs. 92 %, respectively), which is frequently accompanied by dizziness and fatigue [14]. Women, however, are more likely to report certain additional symptoms such as indigestion, shortness of breath, palpitations, mid-back pain, and nausea [14] as well as pain in the jaw, arm, and throat (Table 18.2) [15]. Due to the wider variety of symptoms seen in women with ACS, they are more likely than men to assume that these symptoms have noncardiac causes [15]. Such differences in presentation may account for longer symptom onset to treatment times and increased door-to-balloon times in women with STEMI undergoing PCI [16].
Table 18.2
Presenting symptoms of cardiovascular disease (CVD) in women
CVD presentation in women |
|---|
Chest paina |
Shortness of breath |
Indigestion |
Palpitations |
Mid-back pain |
Pain to jaw, arm, and throat |
Attribute pain to noncardiac causes |
Barriers to Optimal Care
Biological Differences
Biological characteristics unique to women play a role in the diagnosis, treatment, and outcome of CVD. Compared to men, women tend to have lower body surface areas, smaller coronary arteries, increased microvascular disease, and different plaque morphology [17]. Younger women, who have higher rates of mortality than age-matched men with ischemic heart disease, are more likely to have erosion of the coronary plaque fibrous cap with intimal exposure [17]. In contrast, older women and men are more likely to have plaques with disrupted caps and necrotic cores [18]. These factors may increase the technical difficulty of coronary artery bypass surgery (CABG) and may partially explain the higher morbidity and mortality experienced in women after CABG.
Cardiovascular events in women are extremely rare before menopause, but become more frequent with age; by the eighth decade of life, the rate for such events is similar for the two sexes [17]. This suggests possible cardioprotective effects for endogenous estrogen in young women. However, exogenous estrogen in the form of hormone replacement therapy appears to have the opposite effect, and in fact, increases the occurrence of CV events in women. The Heart and Estrogen/Progestin Replacement Study (HERS), a secondary prevention trial, found an increased risk of MI in the first few months after initiation of hormone therapy and an increased risk of venous thromboembolic events [19]. The Women’s Health Initiative (WHI), a primary prevention trial, found an increased risk of stroke in healthy women taking estrogen plus progestin, without benefit in the incidence of CAD [20]. HRT use was not recommended for CVD prevention in either the WHI (primary) or HERS (secondary) trials. Current AHA/ACC guidelines advise that women with known cardiovascular disease not begin HRT and furthermore that those already on HRT should strongly consider discontinuing its use [21].
Diagnostic Testing
Additional diagnostic challenges exist for women with suspected CAD. Higher percentages of women than men with CAD have normal coronary arteries on diagnostic angiography, highlighting the inaccuracies of proper risk assessment for women prior to testing [16–21]. A combination of differences in clinical presentation and the prevalence of coronary disease prove problematic. Recommendations regarding the use of stress testing in women are often derived from studies performed in men, since there is a paucity of data regarding the best diagnostic strategy for assessment of coronary artery disease in women. In the Coronary Artery Surgery Study (CASS), 50 % of women undergoing coronary angiography for chest pain were found to have nonobstructive disease compared to 17 % of men, yet studies show that women without obstruction may continue to experience cardiac symptoms [22–24].
Electrocardiogram (ECG) stress testing is frequently performed in women, with ACC/AHA guidelines recommending it for those at intermediate and high risk for CAD [25]. However, exercise stress tests alone have decreased sensitivity and specificity in women compared to men, which might be explained by lower ECG voltage or endogenous estrogen in premenopausal women and exogenous estrogen in older women [26, 27]. Alternatively, the addition of imaging to exercise stress testing appears to be both cost-effective and accurate in risk stratification for both sexes [25]. Both stress echocardiography and stress gated myocardial single-photon emission computed tomographic nuclear imaging appear to perform similarly in women [25]. SPECT imaging with dobutamine appears to be another diagnostically accurate tool for detecting CAD in women. Cardiac MRI (CMR) may be useful due to its ability to detect stenosis as well as physiological characteristics such as perfusion and metabolism. CT angiography detects coronary calcification and coronary stenosis. This technique has shown high diagnostic accuracy; however, its diagnostic accuracy in women compare to that for men is uncertain remains to be seen [28–30].
Women have lower rates of revascularization [31, 32], noninvasive testing [31], and arteriography [31] than men despite a similar incidence of ACS. In an ACS registry of 20,290 patients, female sex was a significant predictor for a low likelihood of undergoing PCI [33]. In the USA between 1988 and 2004, men underwent multivessel PCI and CABG twice as often as women [34]. Lower frequency of PCI in women has also been seen in France [35] and Korea [36]. In a study of 32,888 patients with evidence of NSTEMI, men were significantly more likely to undergo intervention [37]. Though most evidence shows differing rates of intervention in ACS between men and women, some have found similar frequency of invasive therapy [38].
Time to admission and intervention is not equivalent between the sexes [39]. In one study of STEMI, women were found to have longer intervals between symptoms and intervention, and there are other reports in the literature of delays at each stage of treatment for STEMI [40, 41]. However, a statewide program focusing on improving STEMI treatment times through regionalization resulted in faster times for women, the elderly, and minorities that were similar to the improved treatment times seen in men. Thus, disparities may be lessened through the use of focused initiatives [33, 42].
Social and Educational Barriers
Social factors also influence treatment and intervention in women with CVD. In a survey of 1,024 women, half correctly named CVD as the number one cause of mortality in women. However, most women could not identify other important CVD risk factors, including smoking and hypertension [43]. Women also have lower documented concern about heart disease as a personal health risk [44]. Lower self-perception of risk may lead to lower acceptance of treatment recommendations [45, 46], with women 6 % less likely to accept a physician recommendation for PCI over medical therapy and 7 % less willing to accept CABG over medical therapy [46].
Healthcare Provider Barriers
Healthcare provider behaviors and infrastructure also play a role in gender inequalities. Bias extends to physicians, who are less likely to perceive women’s pain as cardiac [23] and less likely to refer women to cardiac rehabilitation [47, 48]. To date the AHA has published three female specific guidelines (2004–2011) for the prevention of CAD [21]. However, physician knowledge and behavior related to gender-specific guidelines is unknown. A national AHA study of 500 physicians revealed that gender disparities in treatment were largely explained by the provider’s perception of lower CAD risk in women, although the calculated risk is actually similar for each sex [49]. A survey of internists and obstetrician/gynecologists on major barriers towards preventive care identified lack of physician time with patients and low reimbursement for lifestyle change interventions as major reasons for lack of primary prevention education [49]. Restructuring health care systems and reimbursement models may narrow the gender gap in preventive care. Gender-related factors related to barriers of care are summarized in Table 18.3.
Table 18.3
Barriers to optimal cardiovascular disease (CVD) diagnosis and care in women
Barriers to optimal CVD diagnosis and care in women |
|---|
Incomplete understanding of role of estrogen in female CVD |
High prevalence of normal coronary arteries on angiography |
Healthcare provider bias |
Low self-perception of risk |
Outcomes in Women Post-PCI
Since the advent of PCI, women have experienced a higher frequency of adverse clinical outcomes compared to men, including reinfarction, emergency coronary artery bypass graft surgery, restenosis, mortality, bleeding, and vascular complications. Improvements in technology have decreased sex differences in outcomes, but discrepancies remain.
Mortality
Trends
Women continue to experience increased in-hospital mortality after PCI compared to men, but the phenomenon frequently disappears following multivariate adjustment. This can be largely explained by women’s increased age and incidence of comorbidities on presentation, rather than inherent sex differences. In a 25-year, single-center study of 18,885 consecutive PCI at the Mayo Clinic, mortality was reduced significantly between an early group of PCI patients (1979–1995) and more recent group (1996–2004) in both men and women [50]. Such improvements in survival rates have been attributed to newer technology, operator technical experience, and improved medical therapy [51].
Short Term
In recent studies which assess the in-hospital mortality after PCI in UA, NSTEMI and STEMI combined, there was usually no difference between men and women after multivariate adjustment [9, 52, 53], though there were some exceptions [10]. In studies of PCI in UA/NSTEMI alone, female sex was not an independent predictor of mortality after adjustment for other factors [4, 5]. In studies of elective procedures alone, there was also no difference in early mortality between men and women [54].
However, controversy regarding the impact of female sex on in-hospital mortality, post-PCI still persists in studies of STEMI. In some studies, in-hospital mortality was higher in women even after multivariate adjustment [35, 55], yet in others the difference disappeared after adjustment [6, 7]. Some evidence exists for higher in-hospital mortality post-acute myocardial infarction (AMI) in certain female subpopulations, such as women under 50 years of age [56], elderly women within 48 h of STEMI [57], and women over 75 [12]. Furthermore, in the Get with the Guidelines-CAD registry, higher in-hospital mortality for women in the STEMI population remained even after adjustment for baseline characteristics, with a high incidence of early deaths [58]. It is possible that underutilization of treatments for women and delayed reperfusion is responsible for the high incidence of early deaths observed [59]. Thirty-day mortality has also been reported to be higher in women in univariate analysis, but sex was not an independent predictor [10, 60].
Long Term
Despite some evidence of persistent early mortality in women undergoing PCI, sex-specific mortality appears to decrease with time. In trials of consecutive PCI patients as well as in studies of PCI registries, there was no adjusted difference in long-term mortality at 6 months, 1 year [10, 53, 61], 3 years [53], or 5 years [62]. In fact, some studies even found that women had decreased mortality compared to men in long-term analysis [53, 63]. On the other hand, D’Ascenzo et al. recently found that although major adverse cardiac events (MACE) following PCI for ACS are similar in men and women, women had a higher long-term mortality rate in PCI following STEMI (20 % vs. 8.1 % at 5 years) [62].
Drug-Eluting (DES) Versus Bare-Metal Stents (BMS) in Women
Stenting is a safe and effective intervention in women with the ability to reduce rates of restenosis and revascularization [64]. In trials of bare-metal stents (BMS) compared to balloon angioplasty, BMS use resulted in reduced in-hospital mortality and subsequent restenosis [65, 66]. BMS use was also more effective than balloon angioplasty when used in smaller coronary arteries [67]. Due to the lack of sex-specific data published in the earlier years of stenting versus balloon angioplasty, the advantages of stenting were generalized to women [1]. With the advent of drug-eluting stents, restenosis and mortality rates have decreased even further. In a randomized trial of BMS versus DES, there were no differences in stent thrombosis or other outcomes between men and women, although DES as an independent predictor for low risk of repeat PCI in both sexes [68]. Solinas et al. evaluated gender-specific outcomes in a pooled analysis of four trials that randomized sirolimus-eluting stents (SES) versus BMS [69]. They found that women, who comprised 28 % of the combined study populations, had significant reductions in rates of in-segment binary restenosis (6.3 % vs. 43.8 %) and significant reductions in 1-year major cardiac events (P < 0.0001) with SES. Others have found similar in-hospital outcomes between men and women following DES [70], decreased long-term target vessel revascularization (TVR) and MACE in women receiving DES [71], and reduced restenosis in both men and women despite less favorable baseline presentations for women [72].
Restenosis and Revascularization
Data on restenosis in women following PCI is conflicting, with some evidence showing increased restenosis in women compared to men, especially in the short term [73–75]. This is due in part to the small sample size of women in the earlier bare-metal stent (BMS) prospective trials. However, studies of newer DES continue to show promise in women.
Both taxus and sirolimus drug-eluting stents have been associated with favorable outcomes in women. The TAXUS IV trial and the SIRIUS trials revealed reductions in target vessel revascularization (TVR) rates, restenosis, and MACE in both sexes at 1 year. In the TAXUS IV trial (n = 1,314, 27.9 % women) women (7.6 %) and men (8.6 %) had comparable rates of restenosis (P = 0.80). When compared to BMS stents, women treated with TAXUS stents had significant reductions in 9-month restenosis (29.2 % vs. 8.6 %, P < 0.001) and 1-year target lesion revascularization rates (TLR) (14.9 % vs. 7.6 %, P = 0.02), (61.5). In a substudy of the SPIRIT III trial (n = 1,001, women 31.4 %), the XIENCE V everolimus-eluting stent was superior to TAXUS at preventing restenosis in women, confirming the observations seen in men [76]. However, compared to men also receiving the XIENCE V, women did have higher rates of MACE and restenosis.
A recent meta-analysis (n = 156,798, women 31.3 %) found that 30-day target vessel revascularization (TVR) was present in 4.25 % of female patients and 3.83 % of male patients, with male sex significantly associated with a reduction in short-term TVR [75]. Although improvements in stent technology have improved overall outcomes, increased rates of diabetes, aspirin resistance, and smaller vessel size among women may be responsible for higher short-term TVR observed in women [75]. Small artery size has been shown to predict restenosis [74]. Estrogen may also be protective against restenosis [74], but this explanation appears insufficient given the older age of many female PCI patients [77]. Further investigation is needed to see how these factors might play a role in short-term TVR, without extending long-term risk. Long-term outcomes for DES in women are also encouraging. A pooled analysis of DES-treated patients from randomized trials (n = 2,271, women 29.3 %) and real-world registries (n = 7,492, women 32.7 %) found that women with DES had lower TLR than women with BMS stents (11.5 % vs. 22.6 %, p < 0.001) and found no difference in 5-year outcomes between DES-treated women versus men [73]. Findings of similar outcomes in men and women with DES may indicate a trend towards reducing disparities among the sexes in gender outcomes post-PCI [78, 79]. It should be noted, however, that access to DES may not be equal between men and women, and thus, outcomes may still differ by sex [62].
Bleeding and Vascular Complications
Despite progress in reducing MACE in the female PCI population, bleeding and vascular complications remain significant sources of morbidity and mortality post-intervention. Recent analysis combining three PCI trials – REPLACE-2, ACUITY, and HORIZONS (n = 17,034, 4,366 women) – found that female sex was one of six main predictors of non-CABG-related TIMI (thrombolysis in myocardial infarction criteria) major bleeding, which was strongly associated with 1-year mortality [80]. Bleeding complications, including access site hematomas and transfusions, remain higher in women compared to men even after adjustment for baseline factors [9, 10, 52, 60, 76, 81–84]. In a study of consecutive PCI in African-Americans (n = 835, female 443), women had higher adjusted major and minor bleeding than men, despite similar in-hospital outcomes [85]. Still, some evidence remains that bleeding risk is partially attributed to comorbidities. In a substudy of the REPLACE-2 trial, sex was associated with in-hospital bleeds in univariate analysis but not after multivariate adjustment [86]. Given the higher risk of bleeding in women, physicians and researchers must continue to find ways to reduce the occurrence of this adverse outcome.
Progress has been made in lowering bleeding and vascular complications. In a study of 45,987 patients (13,653 women) from the Northern New England PCI Registry, there was a 50 % decrease in absolute bleeding and vascular complications from 2002 to 2007 [87]. Protective factors included fluoroscopy-guided access, use of closure devices, and use of direct thrombin inhibitor bivalirudin [87]. Bivalirudin has been shown to decrease major and minor bleeding in women, including access site bleeding, compared to heparin + glycoprotein IIb/IIIa inhibitor [86]. Similarly, in an 8-year study of CATH and PCI from 1998 to 2005 (n = 18,467, 7,922 women), the vascular complication rate was 3.2 % in women undergoing PCI compared with 1.3 % in men. Up until the last year of the trial, female sex remained a strong predictor of vascular complications. Notably, during the study period the vascular complication rates decreased significantly in both men and women, which might be attributed to the use of smaller sheath sizes in later years [88]. In the large-scale ACC-NCDR registry (n = 199,690 patients, 68,026 women), women retained higher rates of vascular complications even after adjusting for confounders [9]. Possible explanations for increased vascular complications in women include aggressive anticoagulation and smaller vessel size. To date, there has been no gender-specific data on arterial vascular closure devices.
Increased use of radial access for PCI may help prevent access site bleeding [89, 90]. In the Radial versus Femoral access for coronary intervention (RIVAL) trial, 7,021 ACS patients (1,861 women) were randomized to femoral or radial access and similar rates of adverse outcomes were observed. Notably, vascular complications were less common in patients undergoing PCI with radial access [89]. In a study of 3,261 PCI (900 in women), there was no major bleeding in women who underwent radial access for PCI, although an alternative access site was used more often in women than men [90]. Procedure failure might be explained by the presence of smaller and tortuous vessels which are more prone to spasm in women [91]. In another study, men and women undergoing radial access did not differ in MACE after 1 year of follow-up, but female sex remained a predictor of mild to moderate access site hematomas even after baseline adjustment [92].
In summary, short-term in-hospital mortality in women following PCI is largely explained by later presentation with the presence of additional comorbidities, while long-term mortality occurs at similar rates in both sexes. Some increased mortality in women with STEMI may remain. Restenosis and revascularization rates may be higher in women in the short term, but may actually be lower in the long term, and these rates continue to decline in the DES era. Bleeding and vascular complications, while declining in frequency, are still more likely to occur in women; however, these outcomes might be mitigated by the use of bivalirudin or the use of radial access.
Other Outcomes
Despite increased comorbidities at presentation, women and men have similar procedural success rates [81, 82, 93]. In one recent study of STEMI patients, women had lower success rates compared to men (94.7 % vs. 95.9 %, p = 0.002) [12], but other studies have found no difference in STEMI success [7]. In a study in Korea, the opposite was true. PCI success rates were similar in STEMI but were higher in men versus women with NSTEMI [36].
Following PCI, women also have higher incidence of cardiogenic shock [9], contrast-induced nephropathy (CIN) [52], and increased likelihood of a rise in serum creatinine greater than 1 mg/dl rise in creatinine [81]. Female sex has been reported as an independent predictor of CIN in some studies [52, 94]. Other studies have shown only a univariate association between female sex and CIN, thus attributing part of the observed sex disparity to differences in baseline conditions [95, 96]. Although female sex is not one of the variables in the Mehran CIN risk score, it is noteworthy that females experience higher rates of certain comorbidities (e.g., CHF, diabetes) that are included in the model [97].
Treatment of Acute Coronary Syndrome in Women
Invasive Versus Conservative Strategies
Randomized trials have demonstrated the benefit of an invasive strategy over conservative treatment in ACS, but it has been difficult to reach conclusions regarding gender-specific strategies. The current AHA/ACC 2007 guidelines for treatment of NSTEMI/UA distinguish between high- and low-risk women for early invasive versus conservative treatment [100]. Invasive therapy is recommended for high-risk men and women, whereas conservative treatment is recommended for low-risk women (Class I, level of evidence B). Low-risk men did not show significant advantages or disadvantages with early invasive strategy. Medical therapy guidelines are the same for both genders, but consideration should be given to correct dosage for body weight [101]. Notably, the 2011 update contained comorbidity recommendations that may especially apply to women. Treatment decisions should be made similarly in patients with and without diabetes. Regarding renal function, consideration should be given to pre-procedure hydration in order to prevent CIN and caution exercised in the use of renally cleared medications [102].
Though randomized trials uniformly favor aggressive therapy for ACS in men, results in women are mixed [103]. The TACTICS-TIMI 18 trial (n = 2,220, 34 % women) randomized patients to receive early invasive therapy or conservative medical therapy, with a primary endpoint of death, MI, or rehospitalization within 30 days. In women who received early invasive therapy, there were similar reductions in the primary composite endpoint to men, and these observed reductions were further increased after accounting for baseline conditions [38]. In the RITA-3 trial, only men derived a significant benefit from early invasive treatment [104]. Similarly, the FRISC II study, which randomized patients to early invasive or noninvasive treatment, found no difference in MI or death at 12 months for women (12.4 % vs. 10.5 %), whereas men had a significantly reduced incidence of the primary endpoint in the invasive arm (9.6 % invasive vs. 15.8 % noninvasive) [105]. FRISC II found an interaction between early intervention and gender on the occurrence of MI and death (p = 0.008), which indicates a different response of men and women to invasive therapy [105]. Recent data from the Berlin Myocardial Infarction Registry (n = 2,808) for years 2004–2008 also indicate that after baseline adjustment that men (0.41, 0.21–0.78) benefited from early invasive therapy, while women (1.24, 0.53–2.91) did not. Possible reasons for the lack of agreement among trials include differences in the baseline procedure risk for women enrolled in each trial difference in definition of MI among the trials and beneficial use of tirofiban with PCI in TACTICS-TIMI 18 [104].
Treatment of Acute Myocardial Infarction in Women
PCI Versus Fibrinolytic Strategies
AHA/ACC STEMI guidelines for both men and women recommend PCI over fibrinolytic therapy, with door-to-balloon time within 90 min [106]. In the GUSTO-IIb PTCA trial, both men and women who underwent PTCA versus tissue plasminogen activator (tPA) administration experienced lower rates of the composite endpoint (death, nonfatal MI, nonfatal disabling stroke) [107]. For women, 56 events per 1,000 women treated with PTCA were prevented compared to 42 events per 1,000 men [108]. In a subanalysis of the PAMI trial which also randomized AMI patients to tPA or PTCA, women had higher mortality than men with tPA but no significant mortality difference with PTCA. In-hospital survival in women was independently predicted by PTCA [109], demonstrating the efficacy of invasive therapy in STEMI for women.
Studies have also compared PTCA with primary stenting in STEMI patients. In the CADILLAC trial, women who had primary stenting experienced a reduction in 1-year MACE (28.1 % in PTCA to 19.1 % in stenting) and ischemic TVR (20.4–10.8 %, P = 0.002) compared to the PTCA arm [64].
Despite evidence of the effectiveness of invasive therapy for STEMI in women, studies have found underuse of reperfusion therapy. In GUSTO V, angiography and subsequent PCI were less frequently performed in women [110]. Similarly, the Get with the Guidelines-CAD Registry found that women in the STEMI population were less likely to undergo revascularization [59].
Adjunctive Pharmacotherapy
Substantial evidence exists for adjunctive pharmacotherapy in PCI, but women receive medical therapy with lower frequency than men following interventions [111, 112]. A study of Canadian ACS Registry patients found that although women received similar antiplatelets to men at discharge, they received fewer lipid-lowering medications and ACE inhibitors, even after multivariate adjustment [112]. Similarly, analysis from the CRUSADE trial (n = 35,875, 41 % women) found that women were less likely to be discharged with aspirin, statins, and ACE inhibitors than men [113]. Others have found lower rates of beta-blockers [114] or glycoprotein IIb/IIIa inhibitors and clopidogrel [115] at discharge. Still, others have found comparable rates of aspirin, lipid-lowering agents, and beta-blockers at discharge [115]. In a study of secondary prevention in women, beta-blockers and statins were underutilized according to current guidelines for patients with CHD [110–112, 116].
Antiplatelet Agents
Aspirin
Large-scale clinical trials such as the Physician’s Health Study and Primary Prevention Project have demonstrated the benefit of aspirin use in men for the prevention of cardiovascular events, but evidence for primary prevention in women is less robust [23]. A recent meta-analysis including 51,342 women [117] found that aspirin reduces the frequency of MI in men by 32 % but does not significantly reduce MI in women. On the other hand, aspirin was associated with a stroke rate decrease of 17 % in women.
Nevertheless, aspirin remains an integral part of AHA 2011 guidelines for the prevention of CVD in women. A daily dose of 75–325 mg/day is recommended in high-risk women with CHD (Class I, Level of Evidence A) as well as women with diabetes mellitus (Class IIa, Level of Evidence B). If aspirin is contraindicated in high-risk women, clopidogrel should be prescribed (Class I, Level of Evidence B). In healthy women over the age of 65, aspirin therapy (81 mg/day or 100 every other day) is recommended for stroke and MI prevention if the benefits outweigh the risk of bleeding (Class IIa, Level B) [118]. In women under the age of 65, aspirin may be reasonable for the prevention of stroke (Class IIb, level of evidence B), but it is not indicated for MI prevention (Class III, Level of evidence B). For women with atrial fibrillation, daily aspirin is recommended if warfarin cannot be used or if they are at low risk for stroke [118].
Evidence for the use of aspirin in secondary prevention is strong but has not been fully assessed by stratifying according to gender [23]. The Antithrombotic Trialists’ Collaboration saw a significant reduction in cardiovascular events (22 %) after the use of aspirin in patients with CVD or at high risk [119]. In light of the lack of evidence saying that secondary prevention in women is not warranted, it is concerning that aspirin is underused as secondary prevention in women [116].
Aspirin use in women may also be complicated by new evidence suggesting that aspirin resistance is up to four times more prevalent in women than men [23]. In a study of healthy drug naïve patients, women were less likely to respond to platelet inhibition therapy by aspirin and were more likely to have higher platelet aggregation at baseline [120]. Others have found that women have the same level of direct COX-1 pathway suppression as men with aspirin, but may maintain some higher level of indirect COX-1 activation [121]. Further studies on gender-based responsiveness to antiplatelet therapy, including alternative options, are necessary.
For the treatment of ACS, ACC/AHA 2011 guidelines recommend administering aspirin immediately upon presentation for UA/NSTEMI and maintaining therapy lifelong. For patients who cannot tolerate aspirin, clopidogrel should be substituted. Patients receiving initial invasive therapy should receive dual antiplatelet therapy before PCI with ASA + clopidogrel or GP IIb/IIIA inhibitors, with additional anticoagulation administered at the time of PCI. Aspirin is also indicated in conservative therapy, with ASA + clopidogrel given for 1-month to 1-year post-event [102].
Thienopyridines
Clopidogrel
Clopidogrel is utilized as part of dual antiplatelet therapy in CAD patients. In a recent meta-analysis that analyzed studies of elective PCI, UA/NSTEMI, and STEMI [122], clopidogrel was beneficial in reducing composite endpoints as well as MI alone in both sexes. In 8.3 months of treatment with clopidogrel, there were 9 fewer CV events per 1,000 women treated and 12 fewer CV events per 1,000 men treated. It should be noted that clopidogrel added to aspirin therapy raised major bleeding risk by 43 % in women and 21 % in men [122].
Clopidogrel’s ability to reduce MACE in elective PCI was tested in Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Patients were randomized to receive a 300 mg loading dose of placebo, followed by clopidogrel through day 28, and then randomized to clopidogrel or placebo for the remainder of 12 months. Aspirin was given to both groups. Subjects who received long-term clopidogrel therapy experienced a 26.9 % decrease in MACE. Risk reduction rate of death, MI, and stroke at 1 year was 32.1 % in women and 24.5 % in men. Pretreatment did not lower the risk of 28-day events. Risk of bleeding was not significantly increased at 1 year [123].
Prasugrel
Prasugrel, a thienopyridine approved in 2009, has stronger effects and more rapid onset than its predecessor, clopidogrel, which demonstrated superiority to clopidogrel in the TRITON-TIMI 38 trial (n = 3,524, 22 % women) [124, 125]. In subgroup analysis, women demonstrated a 12 % decrease in the primary MACE endpoint., while men revealed a 21 % reduction [126]. Recent subgroup findings of TRITON-TIMI 38 showed that women and men had similar rates of non-CABG TIMI major bleeding, but only women had higher non-CABG minor bleeding [127].
The latest guidelines from the AHA/ACC do not comment on the use of prasugrel in elective PCI, but guidelines for UA/NSTEMI [102] and STEMI [128] were updated to include the administration of 60 mg prasugrel upon presentation in patients with a planned PCI. Those updates also describe patients in whom prasugrel may cause harm: patients with a history of stroke or transient ischemic attack, weight below 60 kg, and age above 75 years. Given the increased likelihood women have of presenting at older age, lower weight, and with more comorbidities, these criteria are particularly relevant for determining appropriate prasugrel use in women.
Ticagrelor
Unlike the irreversible platelet inhibitors prasugrel and clopidogrel, ticagrelor is a reversible P2Y12-receptor antagonist recently approved in the United States. In the Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes (PLATO) trial (n = 18,624, 28.4 % women ticagrelor arm and 28.3 % women clopidogrel arm), there were no differences in the primary composite endpoint of death from vascular causes, MI, or stroke between the sexes for patients who were hospitalized for ACS [129]. However, within each sex, there was a gender-specific risk reduction; low-weight men and women were less likely to benefit from ticagrelor than higher weight men and women.
Notably, ticagrelor approval in the United States was delayed compared to Europe due to increased adverse outcomes in the North American cohort in patients randomized to ticagrelor [130]. The increase in complications has been attributed to a higher dosage of aspirin prescribed in the United States compared to other sites [131]. Upon examination of other sites with high aspirin dosage, similar increased outcomes were noted. The Food and Drug Administration (FDA) has approved ticagrelor with a black box warning regarding concomitant high-dose aspirin.
Substudies of PLATO for ACS patients with planned invasive therapy (both STEMI and NSTEMI) showed similar results [132], with no difference in the primary endpoint between men and women. In an STE-ACS subpopulation of PLATO (n = 7,544; women = 1,796), primary outcomes did not differ significantly between men and women.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree