Chapter 35 Brain tumors are the most common solid pediatric tumors and are the leading cause of death in children from solid tumors.1 The estimated incidence of all childhood primary brain and central nervous system (CNS) tumors is 4.8 cases per 100,000 person-years.2 Approximately 4150 new cases of childhood primary nonmalignant and malignant brain and CNS tumors were diagnosed in the United States in 2011.3 Nearly 50% of brain tumors in children older than 1 year arise in an infratentorial location. However, in neonates, infants, and children up to the age of 3 years, supratentorial tumors are more common.3 The etiology of pediatric brain tumors, an area of research beyond the scope of this chapter, requires an understanding of genetic alterations, signaling systems, and molecular genetics and pathways. Although no one risk factor explains more than a small percentage of childhood brain tumors, therapeutic doses of ionizing radiation to the head for brain tumors and radiation for leukemia,4,5 as well as certain genetic syndromes, are known risk factors in the pediatric population. Among the congenital syndromes associated with brain tumors are neurofibromatosis types 1 and 2, Gorlin syndrome (basal cell nevus syndrome), tuberous sclerosis, Turcot syndrome, von Hippel-Lindau syndrome, and Li-Fraumeni syndrome.6 Because of its superior soft tissue resolution, multiplanar capability, and lack of ionizing radiation, magnetic resonance imaging (MRI) with contrast is the modality of choice in determining lesion size, location, and characterization. And although contrast enhancement typically reflects disruption of the blood-brain barrier, the degree of contrast enhancement does not always correlate with tumor grade. For example, benign tumors (e.g., choroid plexus papillomas and pilocytic astrocytomas) can enhance avidly, whereas anaplastic astrocytomas may not enhance at all.7 MRI also is used to assess tumor response and progression and monitor treatment effects. Essential to optimal treatment planning is accurate staging of the tumor that confirms whether the tumor has spread through the neural axis. Intraoperative MRI is being used in some centers to guide both conventional and minimally invasive tumor resection. As these systems are refined, they are expected to form the standard of care at many medical centers.8 Contrast on diffusion-weighted images (DWI) reflects the mean distance traveled by free water protons in tissue as a result of Brownian motion.9,10 Diffusion occurs freely in the direction of white matter tract orientation and is restricted in orthogonal planes. DWI can assess the properties of diffusion occurring within a particular voxel, which is expressed as the apparent diffusion coefficient (ADC). A markedly decreased ADC usually correlates well with increased tumor cellularity in brain neoplasms. Vasogenic edema and necrosis show an increased ADC.7,11,12 ADC values are interpreted in conjunction with structural MRI sequences. Diffusion tensor imaging (DTI), an adaptation of DWI, acquires diffusion data in six or more directions to establish the direction and magnitude of water diffusion. DWI also can be extremely useful in the postoperative period, when low ADC values at the surgical margins or within the resection cavity may be indicative of ischemia or abscess. This technique typically is used in conjunction with conventional MRI, which helps exclude artifact from hematoma.13 Further, DTI aids in identifying patterns of tumor interaction with white matter fiber tracts (i.e., the extent of deviation, edema, infiltration, and destruction),14 and when used in conjunction with volumetric data, it effectively guides surgical resection and predicts possible postoperative deficits resulting from white matter tract damage (e-Fig. 35-1). Reduced fractional anisotropy (FA), which is a measure of the directional diffusivity of water made using DTI, has been found in the white matter of patients with a medulloblastoma, even in the absence of abnormalities on structural sequences.15 Decreased FA values have been shown to correlate with the age at which radiation was administered and with poor academic performance among school-age patients.16 FA thus may be considered a noninvasive biomarker to monitor effects of radiotherapy.16 e-Figure 35-1 Presurgical tractography in a glioma lesion. Magnetic resonance spectroscopy (MRS) is a noninvasive in vivo technique that provides metabolic information beyond structural imaging sequences. It enables detection and quantification of abnormal metabolites in the brain and can help identify tumor tissue, differentiate tumor types, and separate active tumor from radiation necrosis or scar formation. MRS can be performed with most standard MRI scanners, typically by incorporating either the point resolved spin echo or stimulated echo acquisition mode techniques. Simultaneous acquisition of MRS from multiple voxels increases spatial resolution; this procedure is known as “chemical shift imaging” or MR spectroscopic imaging.17 Most brain tumors are characterized by the presence of increased choline/creatine and decreased NAA/creatine ratios, indicating loss of neuroaxonal integrity and increased cell membrane turnover. The presence of lactate in the tumor suggests an anaerobic process with impaired energy metabolism.18 In general, high-grade tumors have higher choline/creatine and lower NAA/creatine ratios than do low-grade lesions. In rapidly growing malignant tumors, necrotic areas may contain lipid resonances.19 However, in pediatric patients, we frequently (and paradoxically) see elevated levels of choline and lactate in pilocytic astrocytomas, a low-grade tumor.20 The presence of specific metabolites such as alanine (an inverted doublet at 1.44 ppm) in meningiomas (e-Fig. 35-2) and taurine (peak at 3.3 to 3.4 ppm) in primitive neuroectodermal tumors (PNETs) may help narrow the differential diagnosis.21,22 Citrate is a tricarboxylic acid cycle intermediate metabolite that has been described in pediatric brain tumors and is found at particularly high levels in pontine gliomas.23 In one study of grade 2 astrocytomas, citrate was significantly more prominent in tumors that progressed.24 e-Figure 35-2 Magnetic resonance spectroscopy (MRS) in a meningioma tumor. Perfusion-weighted imaging measures cerebral hemodynamics at the microcirculation level. Parameters measured by perfusion-weighted imaging include cerebral blood volume (CBV), cerebral blood flow, and mean transit time. Of these, the CBV, defined as the volume of blood in an area of brain tissue expressed in mL/100 g, is the most commonly used parameter in evaluation of brain tumors.25 Lower grade astrocytomas have relatively lower regional CBV than do higher-grade tumors such as anaplastic astrocytomas and glioblastomas26 (e-Fig. 35-3). However, low-grade pediatric pilocytic astrocytomas can have a high relative cerebral blood volume.27 e-Figure 35-3 Perfusion-weighted imaging in a thalamic glioblastoma multiforme tumor. The most widely available technique is T2*-weighted dynamic susceptibility contrast imaging, which consists of a rapid bolus of intravenous (IV) paramagnetic contrast agent followed by a rapid acquisition of echo-planar images during the first pass of contrast material through the capillary bed. As the contrast medium is delivered, it goes through the tissues and results in a signal drop proportional to the blood volume during the first pass. Routine use of this technique in children requires the use of rapid contrast medium injection and power injectors, as well as strategies to overcome problems associated with large-bore IV catheter placement, especially in infants.28 ASL uses endogenous blood as a tracer. The two major types of ASL, pulsed and continuous, are now widely available on clinical scanners. A third type, pseudocontinuous ASL, has just recently been introduced for clinical use. ASL has shown promise for hemodynamic evaluation of brain tumors,29,30 but data in children are limited at this time. Functional MRI (fMRI) is a technique that essentially relies on two physical principles, namely, that oxyhemoglobin is diamagnetic and deoxyhemoglobin is paramagnetic in nature. Because of the relative increased blood flow and consequent increased utilization of oxygen within the activated portions of the brain, the MR signal, in this case known as the “blood oxygen level dependent signal,” or BOLD, is measurably different compared with other parts of the brain. The primary value of fMRI is in localizing the eloquent areas in the brain controlling language, motor skills, and memory. This information helps provide surgical guidance.31 More detailed information can be found in Chapter 27. The role of PET in the evaluation of pediatric brain tumors is to determine metabolic activity at diagnosis, assess response to therapy, and distinguish treatment effect versus tumor recurrence. Fluorine-18-deoxyglucose (18F-FDG) is the most commonly used isotope for PET studies in children. PET scanning using other labeled agents such as the amino acid analogues [11C] methionine and [11C] tyrosine have shown promise in detecting low-grade tumors in adults, although their diagnostic value in children has not yet been established.32 These amino acid analogues are incorporated via amino acid transport pathways into tumor proteins, and therefore uptake reflects tumor protein synthesis.33,34 Other isotopes still at the investigational stage include cell proliferation agents (e.g., 18F-fluorothymidine) and cell hypoxia imaging agents (e.g., 18F-fluoromisonidazole and 62Cu-labeled diacetyl-bis [N4-methylthiosemicarbazone]). Classification of Pediatric Brain Tumors The differential diagnosis is effectively limited by classifying tumors by location, describing the appearance of the lesion on conventional MRI, and applying advanced imaging techniques (Box 35-1). The modified World Health Organization (WHO) classification of CNS tumors divides astrocytomas into low grade (grades I and II) and high grade (grades III and IV).35 Grading of astrocytomas by the WHO criteria is predictive of patient survival.35 Pilocytic astrocytomas are grade I WHO tumors; they account for 20% to 30% of all childhood brain tumors.36 Pilocytic astrocytomas typically arise in the first two decades of life. The most common locations of these tumors are in the optic pathways, hypothalamus, thalamus, basal ganglia, cerebral hemispheres, cerebellum (Fig. 35-4), and brainstem. Patients with neurofibromatosis type 1 (NF1) have an increased risk of the development of pilocytic astrocytomas, including optic pathway tumors. Patients with NF1 who have optic pathway tumors tend to have a better long-term prognosis than do patients without NF1 who have optic pathway tumors.37 Figure 35-4 A cerebellar astrocytoma. Rarely, pilocytic astrocytomas can present with diffuse leptomeningeal spread, which most often is seen in association with the diencephalic syndrome (discussed later in this chapter) or with the pilomyxoid variant of astrocytomas. Pilomyxoid astrocytomas have an indolent course, but their propensity for slow-growing, persistent recurrences makes them difficult to treat.38 High-grade gliomas in children are significantly less common than are low-grade lesions, which account for up to 20% of all hemispheric gliomas.39,40 On CT, these lesions demonstrate heterogeneous enhancement and density with edema, occasional hemorrhage, mass effect, and ill-defined margins. On MRI, these lesions have heterogeneous signal intensity (Fig. 35-5). They typically are hypointense on T1-weighted images and hyperintense on T2-weighted images with surrounding white matter edema. They show effect of the mass on surrounding structures and demonstrate irregular enhancement with necrosis and hemorrhage similar to that seen on CT. Figure 35-5 A supratentorial high-grade glioma. Aggressive surgical resection with preservation of neurological function, followed by radiotherapy directed at the tumor bed, remains the cornerstone of treatment of pediatric malignant gliomas.41 The addition of chemotherapy has been shown to improve survival compared with surgery and radiotherapy alone.40 The overall prognosis for children with supratentorial malignant gliomas remains poor, however, with 5-year progression-free survival rates of around 30%.40 Although supratentorial PNETs are relatively rare, these tumors are more common in the first decade of life, with peak incidence from birth to 5 years of age.42 They account for 5% of all supratentorial tumors in childhood. At presentation they often are large and fairly well defined, occurring either in the cerebral hemispheres or in the lateral ventricles. They may be solid and homogenous or heterogeneous with cyst formation.43 Calcification often is seen on CT. Heterogeneous contrast enhancement is seen along with regions of necrosis. On MRI, solid areas have restricted diffusion and T2-hypointense areas (Fig. 35-6), reflecting high nuclear-to-cytoplasm ratio, increased cellularity, and increased CBV values. Necrosis and hemorrhage also can occur in these lesions.
Pediatric Brain Neoplasms
Etiology
Imaging
Diffusion-Weighted Imaging
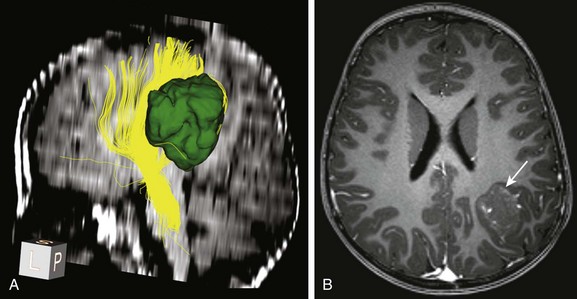
A, Tractography of a portion of the left corticospinal tract forming the posterior limb of the internal capsule (depicted in yellow) in a patient with left parietal lobe angiocentric glioma (segmented and shown in green). The tract is displaced medially by the tumor but is still intact.
B, An axial postcontrast magnetization-prepared rapid gradient-echo image shows the cortically based minimally enhancing tumor (arrow).
Magnetic Resonance Spectroscopy
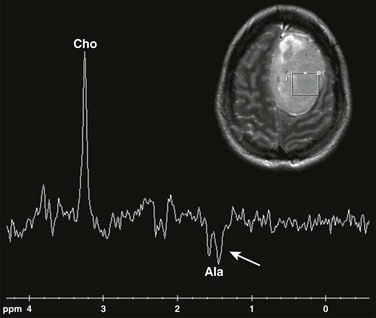
Single voxel MRS (TE 144 ms) performed over the left frontal meningioma (see inset T2-weighted image) shows a prominent choline peak (Cho) and reduced creatine and N-acetylaspartate peaks. The inverted doublet centered at approximately 1.55 to 1.6 ppm is consistent with an alanine (Ala) peak.
Perfusion-Weighted imaging
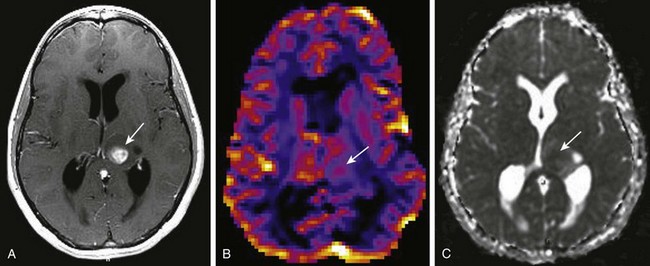
A, A T1-weighted image shows a rounded centrally enhancing mass lesion in the left thalamus (arrow). B, An axial T2* cerebral blood volume perfusion image shows a focal area of increased perfusion corresponding to the enhancing solid portion of the tumor in the left thalamus (arrow). C, An apparent diffusion coefficient map shows regions of decreased diffusion corresponding to the solid component of the left thalamic tumor (arrow).
Functional MRI
Single PET and PET
Specific Tumors
Tumors of the Cerebral Hemispheres
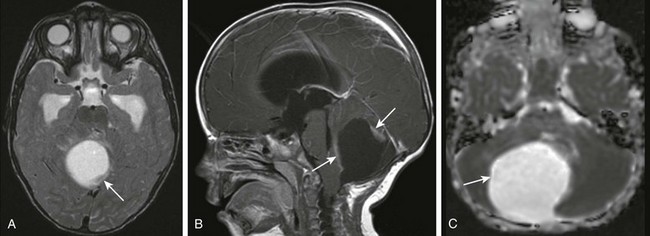
A, An axial T2-weighted image shows a large cystic mass centered in the right cerebellar hemisphere with small solid components along the posterior and anterior aspects (arrow). B, A postcontrast sagittal T1-weighted image shows the solid components (arrows) along the anterior and posterior margins that enhance after paramagnetic contrast administration. C, An apparent diffusion coefficient map shows increased diffusion within the lesion (arrow) consistent with the relatively low cellularity within the lesion.
Supratentorial High-Grade Gliomas
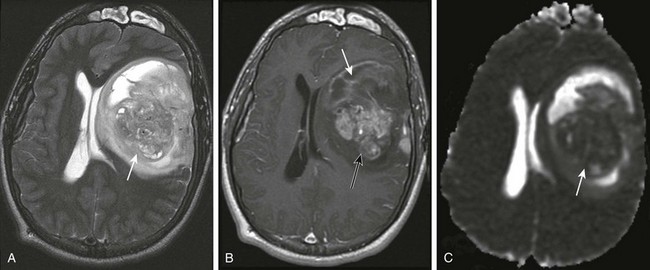
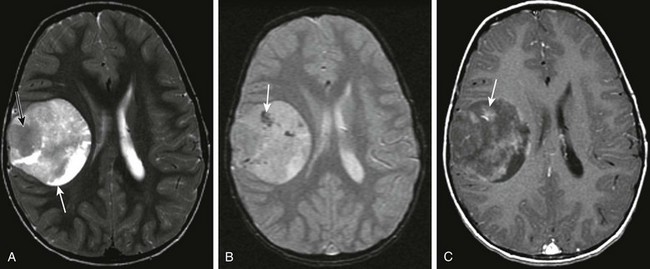
A, An axial T2-weighted image shows a large heterogeneous mass (arrow) in the left frontal, temporal, and parietal lobes with marked mass effect, subfalcine herniation, surrounding edema, and rightward midline shift. B, A postcontrast T1-weighted image shows heterogeneous enhancement of the solid components of the tumor (black arrow) and nonenhancing components anteriorly (white arrow), suggestive of necrosis. C, An apparent diffusion coefficient map reveals decreased diffusion within the solid component (arrow), indicating high cellularity.
Supratentorial PNETs