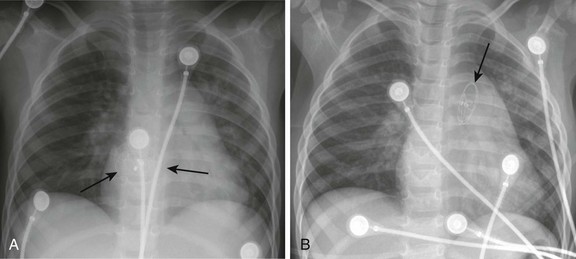
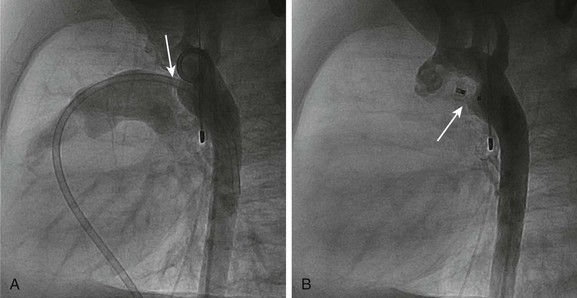
Chapter 69 During the past few decades, noninvasive imaging techniques including echocardiography, magnetic resonance angiography, and computed tomography angiography have evolved significantly in the assessment of complex congenital cardiovascular anatomy. Consequently, use of the catheterization laboratory has evolved to include both diagnostic procedures to assess complex congenital abnormalities before surgery and interventional procedures that permit therapeutic options and often preclude surgery.1 Since transcatheter balloon pulmonary valvuloplasty for valvar pulmonary stenosis in infants was first reported in the early 1980s, it has become the first line of therapy. The recommended balloon/annulus diameter ratio is 120%.2 The reported success rate is greater than 90%, with major adverse events occurring in fewer than 1% of the procedures.3 Hemodynamic measurements include the right ventricular pressure compared with systemic arterial pressure and the peak-to-peak systolic pressure gradient across the pulmonary valve. The indication for balloon pulmonary valvuloplasty is the presence of at least moderate pulmonary valve stenosis. We use as a guideline the “rule of 50,” which is defined as a peak right ventricular systolic pressure of more than 50 mm Hg, a peak right ventricular systolic pressure more than 50% of the systemic systolic pressure, or a peak-to-peak systolic gradient across the pulmonary valve of more than 50 mm Hg. Valvar aortic stenosis can be classified into two groups: disease that is severe enough that it presents at birth or within 1 year of age (10% to 15%), and disease that is not diagnosed until after age 2 years and will progress much more slowly, if at all.4,5 Mortality and the need for intervention are significantly skewed toward the infantile group. As with pulmonary stenosis, noninvasive imaging techniques have advanced to the point that nearly all anatomic and functional information about the valve may be obtained without catheterization. Catheterization is performed for valves that clearly merit intervention or when symptoms and imaging findings are incomplete or confounding. Transcatheter stent angioplasty for postoperative recoarctation of the aorta at the isthmus has been demonstrated to be safe and effective.5 The technique usually involves a femoral arterial approach. An exchange length wire is placed in the ascending aorta or right subclavian artery. An angioplasty balloon is used with a maximum diameter that is equal to or less than the diameter of the normal aortic segments adjacent to the stenotic region and/or the diameter of the descending thoracic aorta at the diaphragm. The stent is mounted on the angioplasty balloon and passed through a sheath at least 1 to 2 Fr larger than that required by the balloon. The stent length is dependent on the lesion length but usually is at least 36 mm in adults. The stent is fully dilated in most cases, but at times it is deemed safer to serially dilate the lesion over two procedures. Although diagnosing the presence of an atrial septal defect (ASD) rarely requires cardiac catheterization, today many patients are undergoing cardiac catheterization for therapeutic device closure.6 These patients require assessment of associated anomalies such as abnormalities of pulmonary venous connections. A step-up in oxygen saturations in the right atrium and pulmonary arteries is characteristic for an ASD, and the degree of left-to-right shunting or the pulmonary to systemic blood flow ratio (Qp : Qs) can be determined. The ideal age or timing for elective ASD closure is 2 to 5 years of age or within 6 to 12 months of diagnosis. Rarely, a child with an ASD presents with severe congestive heart failure and requires intervention in the first year of life. The world experience of transcatheter ASD closure using the Food and Drug Administration–approved Amplatzer Septal Occluder (AGA Medical Corporation, Golden Valley, MN) has been reported with a greater than 97% immediate success rate.7 The occlusion rate reached 100% by 3 years. An overall 2.8% adverse event rate was found, and no procedural deaths occurred. Several studies regarding comparisons of device occlusion versus surgical closure have been performed prospectively. These reports include the Amplatzer and Helex (W.L. Gore & Associates, Flagstaff, AZ) devices for ASD closure. Findings include shorter hospital stays, less discomfort, and shorter durations for convalescence in patients undergoing successful closure with use of a device. Hospital costs are similar. Regression of right ventricular dilatation was similar for both groups of patients; however, it was dependent on the patient’s age at the time of closure, with greater regression following earlier intervention. Procedural adverse events are uncommon but include embolization into the right or left atrium, pulmonary artery, left ventricle, and aorta; stroke as a result of a clot or air embolization; and bleeding complications.8 Both acute and late embolization have been reported, and thus it is critical for the radiologist to be familiar with the location of the interatrial septum on radiography to ascertain correct device position in the anteroposterior and lateral chest radiograph. Figure 69-1, A, demonstrates a septal occluder device in the appropriate position within the interatrial septum, and Figure 69-1, B, demonstrates the device positioned incorrectly in the left pulmonary artery. The first transcatheter interventional procedure was closure of a persistently patent ductus arteriosus (PDA), which was performed by Portsmann in 1967. Since that time a number of different PDA closure devices have been studied, some of which are no longer available. The goal of the procedure has always been to achieve 100% closure of the PDA without obstruction of either adjoining blood vessel (i.e., the aorta and the left pulmonary artery) with minimal risk of complications. The difficulty is that the PDA exhibits extreme variability in size and shape. Krichenko et al.9 classified the PDA into five anatomic types based on the lateral aortic angiogram. The most common (“type A”) ductus is conical with a narrowed pulmonary arterial end and large aortic ampulla. Other types include those with a narrowed aortic end, narrowing at both ends, and a tubular configuration. Early device closure procedures were complicated by a lack of choices for vessels of different sizes and shapes. The early devices had unsatisfactory rates of complications and residual leaks and were abandoned. In 1992, the first report was published using the Gianturco embolization coil (Cook Medical, Bloomington, IN) for closure of small PDAs. The Gianturco coil, which is available in multiple lengths and diameters, has now been in use for more than 20 years for blood vessel occlusion, including unwanted collateral vessels, fistulae, and arteriovenous malformations. The successful use of the coil in PDA closure, combined with sharing of ingenious techniques to deploy multiple coils and secure the coils before deployment by individual operators, provided the needed variety of approaches for successful PDA closure. At present, tens of thousands of patients around the world have had PDAs closed with embolization coils. A more recent addition to the interventional cardiologist’s armamentarium for PDA closure is the Amplatzer Duct Occluder.10 The PDA occluder is a self-expanding wire mesh device that is attached to a delivery cable and deployed through a long sheath from the venous system. Figure 69-2, A, demonstrates a lateral projection angiogram of the descending thoracic aorta and a left-to-right shunting PDA. Figure 69-2, B, demonstrates device occlusion of the PDA. The device has a retention skirt to occupy the aortic ampulla and tapers slightly to the pulmonary artery end. The device is filled with a polyester mesh that stimulates thrombus formation within the lumen of the PDA. The shape of the device and self-expanding properties exert radial force on the walls of the PDA, holding the device in place until endothelialization occurs. The device has excellent closure rates approaching 100% at 1 month after the procedure. The main limitation is the size and bulky nature of the device. The PDA must have an aortic ampulla adequate to accommodate the retention skirt without creating aortic obstruction. Furthermore, the device can create left pulmonary artery stenosis by compressing adjoining structures once it is released.
Pediatric Cardiac Catheterization and Electrophysiology
Pediatric Cardiac Catheterization
Semilunar Valve Stenosis and Balloon Valvuloplasty
Balloon or Stent Angioplasty
Septal and Vascular Occlusion Devices
Pediatric Cardiac Catheterization and Electrophysiology