Sergio Sierre
BACKGROUND
Endovascular techniques used in children for diagnostic and therapeutic purposes are relatively similar compared to those used for adults. Most of these procedures performed in the pediatric population have been extrapolated from the adult experience. However, vascular interventional radiology in pediatrics differs from adult vascular interventions in several aspects, and we frequently face the need for miniaturization of equipment for appropriate use in the pediatric population. Despite anatomical and technical issues, significant clinical and technical success rates have been achieved with endovascular techniques in the pediatric population.1
Furthermore, when the patient is evaluated for moderate sedation or general anesthesia, it is important to consider the maintenance of body temperature control, fluid balance, iodine contrast dose, radiation safety, and equipment selection. Also, technique standardization of any angiographic/vascular procedure is limited due to the fact that different devices are necessary to perform procedures safely in patients with a large range of body size. Children have both advantages and disadvantages and may require alternative techniques. Complications may be similar to those reported in adults, but specific problems may be seen with different prevalence (i.e., vessel thrombosis).1,2
There has been tremendous growth in pediatric vascular interventions, and most recently, pediatric embolotherapy has become technically feasible mainly due to the availability of smaller diagnostic catheters and microcatheters. This chapter will focus on some specific indications for embolotherapy in pediatrics, emphasizing particular issues to be considered in this age group.1–3
Patient Preparation
For all procedures, informed consent is obtained from the parent, guardian, or the patient if they are of legal age to consent. In these forms, the procedure is described, therapeutic alternatives are discussed, and procedure-related complications are mentioned. Routine blood work and coagulation studies are requested. For therapeutic procedures, the coagulation references are platelet count over 50,000/µL, prothrombin time less than 18 seconds, partial thromboplastin time less than 32 seconds, and international normalized ratio less than 1.2. If the blood tests are abnormal, coagulation has to be optimized to avoid procedural bleeding, and a consultation with a hematologist is recommended. Patients are not routinely blood matched, except for those procedures when a possibility of significant blood loss exists, such as transarterial or transjugular intrahepatic portosystemic shunt procedures in coagulopathic patients.4
Patient’s diet is held for an appropriate time as determined by age, alimentary requirements, type of sedation, and need for general anesthesia. Regarding diet, solids may be consumed until 8 hours before sedation or general anesthesia in 6 months of age or older patients. In younger patients, breast milk and formula are permitted until 4 hours before the procedure. Also, patients are able to take clear fluids by mouth up to 2 hours before the procedure. Antibiotics are not routinely prescribed before the embolization procedure, with particular exception for splenic embolization and patients with congenital heart defects. For the former, we usually use a recommended broad-spectrum antibiotic regimen (ceftriaxone/clindamycin) just before and for 5 days after the embolization procedure. For the latter, we use a prophylactic single dose of cefazolin or cefotaxime, 50 mg/kg.2,4
In all cases, the angiography room must be warm, particularly when procedures are performed in newborns and infants. Patients must be at a constant body temperature, and covers, hat, Bair Hugger (Arizant Healthcare, Inc., St Paul, Minnesota), heat lamp, and warming blanket are frequently necessary. Also, any solutions used including the contrast medium should be slightly heated. In pediatrics, the amount of contrast media is a limiting factor. The accepted dose of nonionic contrast should not exceed 5 mL/kg.1–4
Sedation/Anesthesia
A sedation protocol is essential to establish a successful pediatric interventional practice. The choice of whether to use conscious sedation or general anesthesia is influenced by many factors, but for embolization procedures, general anesthesia is preferred.3,5
When planning for sedation, special consideration should be given to children who are very ill, especially those with hepatic and/or renal failure. These patients are at greater risk for acute drug toxicity, hypotension, respiratory depression, and prolonged sedation because of their inability to metabolize and excrete most sedation drugs. Therefore, general anesthesia is often preferred for this group.2–4 General anesthesia is also preferred when the procedure is expected to be lengthy or painful, when there is a history of failed or difficult sedation, or when there are contraindications or unacceptable risks to sedation.3 Hence, general anesthesia is usually indicated for embolization procedures.1–5
Radiation Safety
Children are more radiation-sensitive than adults and have a longer lifespan during which to manifest radiation-induced cancers. Therefore, it is prudent and important to use appropriate radiation safety techniques when performing pediatric interventional procedures.6 Radiologists, radiologic technologists, and all supervising physicians have a responsibility to minimize the radiation dose to individual patients and to staff while maintaining the necessary diagnostic image quality, following the “as low as reasonably achievable (ALARA)” principle. Vascular procedures might be associated with a moderate radiation dose. Also, lengthy or repeated procedures may result in significant radiation exposure, making it extremely necessary to respect and follow the radiation safety protocols. Examination protocols should vary, taking into account patient body habitus, such as height and/or weight, body mass index, or lateral width. The dose reduction devices that are available on imaging equipment should be active; if not, manual techniques should be used to moderate the exposure while maintaining the necessary diagnostic image quality.7
Some techniques for decreasing patient radiation dose should be considered, using reduced-dose protocols, the last-image-hold feature, pulsed fluoroscopy, tightly collimating to the area of interest, and minimizing the use of magnification. Periodically, radiation exposures should be measured and patient radiation doses estimated by a medical physicist in accordance with the appropriate technical standards.5–7
Vascular Access
Vascular access in children based on palpation and anatomic landmarks can be challenging. Ultrasound (US) guidance for the arterial puncture may increase the chances of successfully gaining access to these small-caliber arteries.5,8 In children weighing more than 30 kg, femoral access is obtained with use of an 18-gauge needle, and a 4-Fr or 5-Fr catheter is inserted. In children weighing between 10 and 30 kg, a 20-gauge needle might be used for arterial puncture and a 4-Fr catheter is used.
Arterial puncture in children weighing less than 10 kg can be technically challenging and should not be done with anything larger than a 20-gauge needle. For smaller children, the use of 3-Fr devices is recommended.4,5 Vascular sheaths are placed in all patients because these procedures are often lengthy and can require catheter exchanges. To prevent vessel thrombosis, an initial bolus of heparin is given (75 to 100 units/kg). In case of lengthy procedures, a subsequent heparin injection should follow. Arterial spasm is frequent in this population and may be treated with intra-arterial injection of papaverine (1 mg/kg) or nitroglycerin (2 to 3 units/kg).1,2,4,5
Embolic Agents
Embolic agents used in pediatric embolotherapy are the same as those used in adult patients. Cautions should be considered in case of alcohol use. A maximum dose of 1 mL/kg (or 60 mL) per session should never be exceeded.1,2 Microcatheters allow practitioners to perform many of these pediatric embolization procedures, and coaxial systems (guide catheter/microcatheters) are ideal for this purpose.
Indications
Diagnostic arteriography is gradually being replaced with noninvasive imaging. Indications for diagnostic vascular studies still occur, but frequently, these diagnostic studies are done in conjunction with a therapeutic intervention (i.e., embolization).
Transcatheter embolization is currently a common procedure in pediatric tertiary centers performed by physicians trained in endovascular procedures and aware of technical modifications and alternatives needed for this age group of patients.1,4,5
It is important to ensure the relevance of the procedure and decision must be taken based on a multidisciplinary approach. This chapter will focus on some particular entities almost exclusively found in pediatric patients, such as vascular tumors and congenital shunts and fistulas. Vascular malformations, a pathology very frequently found in pediatric interventional radiology practice, will not be discussed in this chapter (see Chapter 13).
VASCULAR ANOMALIES
In 1982, Drs. Mulliken and Glowacki proposed a classification of vascular congenital anomalies based on the clinical, biologic, and cytologic findings. According to this classification, vascular anomalies were divided into either vascular tumors (hemangioma, hemangioendothelioma, and other vascular tumors) or vascular malformations (capillary, venous, lymphatic, arteriovenous, and combined lesions).9,10
Vascular malformations are composed of irregularly dilated, malformed, or dysplastic vessels, with variably thickened vascular channels lined with mature endothelial cells. These lesions are usually present at birth and do not regress. Vascular malformations exhibit a predictable group of clinical patterns that vary in severity and rate of progression. These vascular malformations were named and classified based on the affected vascular channel (arterial, capillary, venous, or lymphatic or in combination).9–11 Later, a complementary hemodynamic classification was described in 1993, dividing vascular malformations into high-flow and low-flow lesions.12
Sclerotherapy is the technique used to treat low-flow vascular malformations. High-flow lesions are treated with superselective transarterial, direct, and transvenous access, with flow reduction techniques, to deliver an adequate dose of sclerosant and embolic agents to the malformation nidus.9–11,13
Vascular tumors demonstrate rapid neonatal growth, endothelial hypercellularity, increased cellular turnover, and in the case of infantile hemangiomas, a later involutive phase. Hemangiomas are the most frequent tumors in infancy.10,11,13,14
Main indications of embolotherapy in vascular tumors are hemangiomas refractory to medical treatment, hemangioendotheliomas with Kasabach-Merritt phenomenon, and liver hemangioma with cardiac failure.1,2,10,11,14
Vascular Tumors
The most frequent vascular tumors in infancy are infantile hemangiomas. Congenital hemangiomas (noninvoluting congenital hemangiomas [NICH] or rapidly involuting congenital hemangiomas [RICH]), hemangioendotheliomas, tufted angiomas, and sarcomas are other vascular tumors seen in children.10,11,14
Hemangiomas
Hemangiomas are the most common tumors in infancy, with predominance in the females (3:1). Generally, they appear in the first week of life, and 60% of the cases are located at the head and neck. As mentioned, the classic infantile hemangioma presents a first stage of rapid proliferation of approximately 3 to 12 months of duration, with a period of stability and a stage of slow regression lasting from 2 to 10 years. Frequently, nearly 80% of these tumors do not need treatment.
The diagnosis of the hemangiomas is mainly clinical. Doppler US and magnetic resonance imaging are useful diagnostic imaging methods, demonstrating the hypervascularity and extension of these tumors. Angiography and the endovascular techniques are only reserved for therapeutic purposes.2,10,11,14
Hemangiomas Refractory to Treatment
Hemangiomas will only need treatment in 10% to 20% of cases, such as periocular hemangiomas with orbital compromise and/or vision impairment; visceral hemangiomas associated with cardiac insufficiency; hemangiomas with persistent ulceration; hemangiomas compromising the airway; facial hemangiomas with rapid growth and distortion, with presumption of important sequels; symptomatic muscular hemangiomas; and in the presence of Kasabach-Merritt phenomenon.
In case a hemangioma needs treatment, medical treatment is usually the first option. The most common first-line therapy is propranolol (2 to 3 mg/kg/day) that typically has excellent results. Other treatments include steroids, interferon, and vincristine.10,14–16
Embolization and/or surgery are required when medical alternatives are ineffective, mostly in cases of liver hemangiomas with cardiac failure, hemangioendothelioma complicated by Kasabach-Merritt phenomenon, and uncontrolled proliferative hemangioma with functional disorder that do not respond to pharmacologic treatment.10,17,18 Embolization of hemangiomas imposes a precise, distal, intratumoral occlusion. Particulated agents (gelfoam and 300 µm microparticles of polyvinyl alcohol or acrylic microspheres) are usually employed for this purpose.17,19
Patients’ weight is a bounding factor in the use of contrast media in these procedures. Therefore, in some cases, a first procedure will be focused on the embolization of main tumor-feeding vessels. If symptoms persist, this procedure can be repeated.1,2,5,17
Kaposiform Hemangioendothelioma
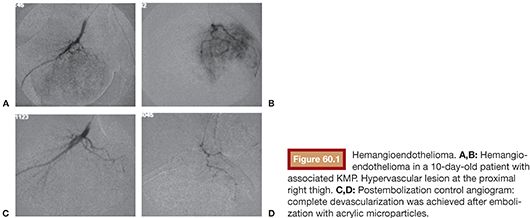
The Kasabach-Merritt phenomenon (KMP) consists of severe thrombocytopenia, microangiopathic hemolytic anemia, and localized consumption coagulopathy, in association with a rapid tumor growth, with reported death rates of 20% to 30%. KMP is actually related to another type of vascular tumor (kaposiform hemangioendothelioma, tufted angioma) and not to the “classic” hemangioma. These tumors present a more invasive and aggressive behavior. Hence, the KMP phenomenon, due to the associated high death rate, needs to be treated.2,11,17,18 Medical treatment is recommended. Steroids are the first-choice treatment, keeping interferon and vincristine for steroid-refractory treatment. Embolization is needed if medical treatment fails and is always associated with medical treatment.2,10,14,18 Embolization, performed by arterial approach, aims at reducing the high flow. In cases of KMP, a distal intratumoral occlusion (Fig. 60.1A–D) is also necessary; therefore, particles are also frequently used in these cases.2,10,15,17,18
Hepatic Hemangiomas
No treatment is required in case of asymptomatic hepatic hemangiomas, but these cases should be closely followed. The main indications for treatment of hepatic hemangiomas are congestive heart failure or KMP.15–17,19 Again, if treatment is needed, medical treatment with propranolol is a first-line therapy. Other treatments include steroids, interferon, and vincristine.10,14–16
In nonresponding patients or in cases where an emergent endovascular treatment is indicated due to a rapid and deleterious progression, embolization is indicated.17 When a hepatic hemangioma needs treatment, a complete vascular mapping is necessary, identifying the arterial vascularization of the tumor (hepatic and extrahepatic feeders) and the patency of the portal vein.19 Different patterns of angiographic findings have been described for hepatic hemangiomas.19 Embolic material is selected depending on the vascular pattern. The most classical appearance is an early filling of abnormal vascular channels without evidence of direct shunting. Others may show high-flow nodules without direct shunts. Embolization is performed by arterial approach for these types, and large particles (≥500 µm) can be used for this purpose. Fatal complications have been reported with the use of small-caliber particles in such cases due to migration to the pulmonary circulation. Hepatic necrosis has also been described with small particles (<300 µm).17,19,20 The choice of the embolic agent is based on the size of the shunts. Also, in small and ill patients, the amount of fluid needed for the delivery of the embolic agent should be minimized. There are no specific recommendations about “contrast:saline solution” for particles infusion; this solution should maintain enough radiopacity to be clearly seen under fluoroscopy. We usually use a 70% contrast/30% saline solution for this purpose.
Hemodynamic patterns including arteriovenous shunts, portovenous shunts, or both arteriovenous and portovenous shunts have been described. Embolization with platinum microcoils are generally safe in such cases and permit the occlusion of the shunts. Glue (N-butyl cyanoacrylate) is also an effective material in patients with direct arteriovenous and arterioportal shunting arising from multiple sources.17,19,20 Glue dilution is based on shunt flow. For shunts involving arterial feeders, a 50:50 (glue:Ethiodol) or greater solution is preferred. Whenever possible, flow reduction techniques might be considered.
Staged procedures are prudent to minimize the appearance of complications such hepatic ischemia, hepatic necrosis, or death.17
Knowing the portal involvement in tumor supply is extremely important for embolization planning. Arterial embolization in cases of portovenous fistulas might be ineffective in reducing the cardiac symptoms and may induce hepatic necrosis. In such cases, embolization of portovenous fistulas from transhepatic or transvenous approach is advocated. If necessary, after the venous occlusion, the arterial embolization may be considered. Clinical treatment (propanolol, steroids, and or vincristine) should be maintained after embolization until nearly complete regression of the lesions.2,17,19,20 The differential diagnosis of liver hemangiomas includes mesenchymal hamartoma, hepatic angiosarcoma, hepatic epithelioid hemangioendothelioma, or metastatic disease, such as neuroblastoma.10,17,20
CONGENITAL PORTOSYSTEMIC SHUNTS
Congenital portosystemic shunts are rarely seen. These abnormal communications can be congenital or acquired, asymptomatic (frequently diagnosed incidentally), or diagnosed due to the presence of shunt complications. Most of them occur in infants.21,22 These shunts are related to the lack of complete involution of one or several fetal vessels, establishing abnormal vascular communications between any vein of the portal system and any vein of the inferior vena cava system. They may exist inside or outside the liver, may be single or multiple, and vary in size. These anomalous communications can cause a partial or complete diversion of the portal flow to a systemic vessel.23 Some small intrahepatic portosystemic shunts located between the portal branches and hepatic veins may resolve spontaneously by age 1 to 2 years; others, mostly the large shunts, such as extrahepatic, persistent ductus venosus, or still patent intrahepatic shunts, persist throughout life and carry risks of complications. They differ from the acquired intrahepatic and extrahepatic portosystemic shunts occurring as a consequence of portal hypertension.24
Associated risks of severe complications exist including neonatal cholestasis, benign and malignant liver tumors, hepatopulmonary syndrome, portopulmonary hypertension, and encephalopathy. The presence of an anomalous portosystemic shunt should be considered in patients with central, not cardiogenic, cyanosis and normal thoracic imaging results (radiography and/or computed tomography [CT]). Hence, the severity of some of these complications and the potential reversibility after shunt occlusion make necessary the treatment of these abnormal communications.23–29
The diagnosis of these entities may be obtained with US and Doppler US imaging, allowing identification of the anomalous communication.
Once a congenital portosystemic shunt is found in a child, either during the investigation of a complication during the neonatal period or later as a fortuitous finding, the first step is to be sure that the shunt is not the consequence of portal hypertension or, during early infancy, of a liver hemangioma that would require a specific treatment.23,24
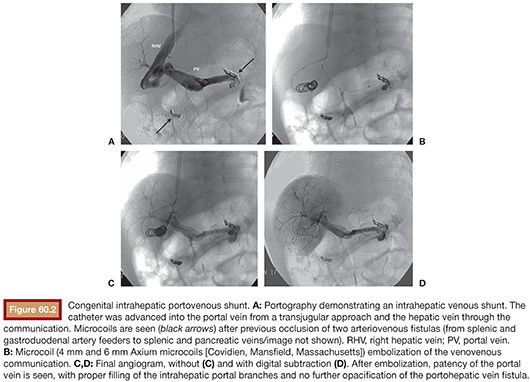
Congenital portosystemic venous shunts are best classified into intrahepatic and extrahepatic varieties. In the former, the connections are created between branches of the portal vein, after its division, and the hepatic veins or inferior vena cava. In extrahepatic shunts, the anastomoses are established between the portomesenteric vasculature, before division of the portal vein, and a systemic vein.21–24,30,31 Therefore, these shunts are divided into two broad types: (1) intrahepatic shunts located between the portal vein or one or several of its branches on one side and the inferior vena cava or a hepatic vein on the other, including the ductus venosus (Fig. 60.2A–D), and (2) extrahepatic shunts, which join directly the portal trunk or one of its branches of origin to the inferior vena cava or one of its branches.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree