Juvenile idiopathic arthritis
Septic arthritis
Other unique disorders in childhood
Transient synovitis
Legg–Perthes–Calvé disease
Slipped upper femoral epiphysis
Acute and chronic recurrent multifocal osteomyelitis
Enthesopathy and apophysitis in sport activities
Musculoskeletal Ultrasound Imaging in Children: Indications and Techniques
Screening for developmental hip dysplasia [6] was the first indication of musculoskeletal US in children. Gradually, the role of musculoskeletal US in standard clinical practice has expanded aiming at improving the care of children with musculoskeletal disorders, particularly juvenile idiopathic arthritis (JIA) [1, 7]. Both US and magnetic resonance (MR) imaging are the two most suitable tools used for the examination of pediatric patients’ immature joints. However, it is important to keep in mind that these methods are complementary rather than exclusive, as they should not replace or precede clinical evaluation. From a pediatric rheumatology perspective, the principal indication of US is direct visualization of joint and tendon sheath inflammatory changes which can be assessed in few minutes, even in the absence of abnormalities on clinical examination [8, 9]. The particular advantage of US over MRI is its ability to scan multiple joints in a single session, which is of paramount importance for the current JIA classification criteria [10]. However, in contrast to its ability to assess peripheral joints, it is not applicable for axial involvement, including the temporomandibular joint [11–13] . In addition to its diagnostic role, US seems to have a promising role in monitoring disease activity and therapeutic response [14], though prognostic evaluation has not been defined yet.
Regarding technical considerations, high-resolution equipment is essential for pediatric musculoskeletal work. As in adults, the choice of transducer (high/low-frequency transducer) will depend on the type of examinations likely to be undertaken. Nevertheless, for pediatric musculoskeletal examination, high-frequency (7.5–20 MHz) linear transducers are generally equally suited in demonstrating superficial structures such as tendons, ligaments, and small joints, as well as larger or deeper joints (Fig. 9.1). Color and Power Doppler techniques provide information on the vascularization of the synovial and soft tissues reflecting vascular abnormalities and active inflammation. However, Doppler setting should be carefully considered due to the finding of some Doppler signals that can be detected at cartilaginous epiphyses as a physiological finding [15]. The size of the footprint (i.e., the surface area of the transducer in contact with the skin) is also an important factor in examination technique. In children, transducers with a large footprint are often inadequate for child’s joint as they cannot be maneuvered adequately. A transducer with a smaller footprint, such as the hockey stick transducer, allows better angulations between the small joints of the hand and the foot, reducing the risk of artifacts.
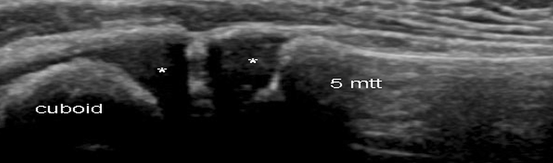

Fig. 9.1
Healthy feet in a 3-year-old child. Longitudinal US scan over the cuboid fifth metatarsal joint showing cuboid and metatarsal bones partially ossified. US depicts this small joint well-defined, epiphyseal hyaline cartilage of those bones appears as anechoic structure (star)
US Imaging: Appearance of the Growing Skeleton
Children differ significantly from adults with respect to skeletal anatomy and physiology. In children, the end of each long bone is composed of the metaphysis, physis (growth plate), and epiphysis. Whereas the skeleton of an adult person is completely ossified except for the articular cartilage, a varying degree of epiphyseal hyaline cartilage is present in addition to the articular cartilage in children. Since the growth cartilage contains a high amount of water; it usually appears as anechoic structure (Fig. 9.2). The US appearance of the joint cartilage in children seems thicker than in adults because the individual components of the joint cartilage (articular and growth cartilages) are initially continuous with each other. They are usually displayed as the only anechoic structure, particularly in early ages. Depending on the age of the child, the sonographer will encounter a variable view of the joint with regard to hyperechoic (cortical bone) and hypo or anechoic portions (epiphyseal cartilage) [17]. However, this also depends on skeletal maturity. Sometimes, the outline of the epiphyseal cartilage can be visualized as hyperechoic line depending on the angle of insonation. A particular feature of the growing skeleton that can also be seen on US is the presence of one or more secondary ossification centers in the epiphyses of the long bones and within the short bones. At birth, cortical bone is present in most long bone diaphyses representing the primary ossification center while many ossification centers in short bones, for example in the wrist, are not present and hardly any epiphyseal bone is present on imaging with radiographs. Since imaging appearance of multiple secondary ossification centers may cause confusion with fragmentation observed in traction apophysitis, it is important to have a deep knowledge of the childhood anatomy. However, there is a paucity of data on the joints-specific imaging features present during growth and development in healthy children [18].
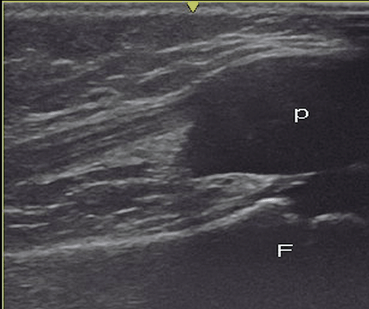

Fig. 9.2
Healthy knee in an 8-month-old child. Longitudinal US scan over the anterior surface of the patella shows the patella (p) as an unossified structure located anterior to the distal epiphysis of the femoral condyle (F). The immature skeleton usually appears as anechoic structure because the growth cartilage contains a high amount of water
Currently, the pediatric subgroup of the Outcome Measures in Rheumatology Clinical Trials (OMERACT) Special Interest Group for musculoskeletal US has published definitions for the individual components of the healthy joint on B-mode US (or gray-scale US) that will support the interpretation of US findings in children having arthritis. The definition for the growing hyaline cartilage on B-mode describes it as a well-defined hypo/anechoic structure that is noncompressible and in which surface can (but does not have to) be detected as a hyperechoic line with evenly spread brighter echoes reflecting vascular channels.
Juvenile Idiopathic Arthritis
JIA, the most common chronic rheumatologic disease of childhood, includes a group of clinically heterogeneous arthritis of unknown etiology presenting before the age of 16 years and lasting for at least 6 weeks [10]. It relies heavily on physical examination to determine the disease activity per current validated definitions [19], although the detection of joint swelling and limited range of motion in pediatric patients varies significantly among experienced clinicians, especially in the small joints of the hand [20, 21]. US has demonstrated higher sensitivity for detecting joint involvement compared to clinical examination [8, 22–25]. The presence of joint involvement in JIA may be expressed by ultrasonographic findings on B-mode such as synovial hypertrophy, effusion, bone erosions, and enthesitis.
Definitions of JIA pathology have varied in different US studies as a recent systematic review showed [14]. The current tendency is to apply the definitions of pathological US findings provided for adult rheumatoid arthritis (RA) by the OMERACT US Group [26]. Effusion and synovial hypertrophy can easily be imaged in peripheral joints. In children, the real-time (or dynamic) examination is the most reliable method of scanning for distinguishing synovitis from epiphyseal cartilage. Effusion is defined as compressible and displaceable, synovial hypertrophy as poorly compressible whereas the epiphyseal cartilage is not (Fig. 9.3). Although joint effusion is an early indicator of active joint disease [27], it is nonspecific. Additionally, effusion, as small as 1 mL, can be detected with US and may represent only a physiological finding in some joints [28]. It is important to keep in mind that there is no effusion-specific imaging features for different diseases or supporting a specific diagnosis. US cannot accurately differentiate whether a fluid collection is inflammatory, infectious, or hemophilic arthropaty. Aspiration of fluid, which is more successful with US guidance, remains the key for the diagnosis of joint disorder. The thin synovial membrane is undetectable under normal circumstances but it can be detected as a hypoechoic structure (relative to adjacent hypoechoic tissues) in case of synovial hypertrophy. US can be useful in monitoring disease activity and evaluating any response to therapy based on the assessment of volume and distribution of the pannus as well as synovial vasculature [29–31].
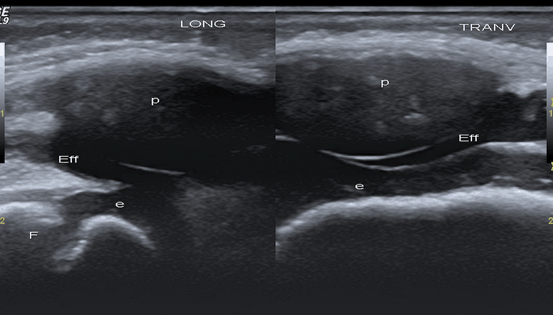

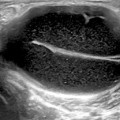
Fig. 9.3
Knee joint in an 8-month-old child. Longitudinal (left side) and transversal (right side) US images of the knee joint. Besides effusion (Eff) within suprapatellar bursa, effusion is visible under the unossified patella (p). Surfaces of the growth cartilage of patella and femur are detected as hyperechoic lines (interface sign) allowing to detect fluid. Dynamic examination would prove that effusion is compressible and displaceable tissue, whereas the epiphyseal cartilage (e) is not
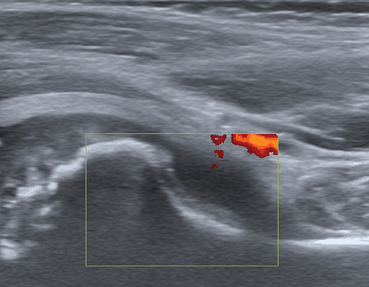
Tendon sheath widening is the hallmark of tenosynovitis that can be detected by US in JIA patients, particularly in the ankle and wrist joints [32–35]. The US appearance of tenosynovitis is the same in patients of all ages [26]. Studies focusing on healthy children depicted the presence of small amount of fluid within tendon sheath as a physiological finding [28]. Color and Power Doppler techniques provide information on the vascularization of the synovial and soft tissues reflecting active inflammation and vascular abnormalities (Fig. 9.4;[31]).
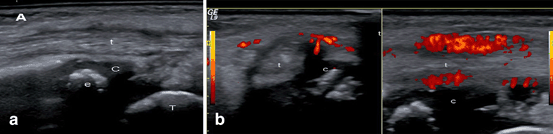

Fig. 9.4
Tenosynovitis of the posterior tibialis tendon. a B-mode US image: Longitudinal US image shows tenosynovitis as the presence of anechoic synovial fluid surrounding the tendon and loss of the homogeneous fibrillar pattern of the tendon (t). C: cartilage of the distal end of tibia. e: secondary ossification center in tibial epiphysis. b Power Doppler US images: Longitudinal and transversal US images showing active inflammation
Entheses inflammation is a distinctive feature of enthesitis-related arthritis (ERA), which is associated with human leukocyte antigen (HLA) B-27 and represents nearly 20 % of JIA categories [36]. Typically, involvement of the lower limb enthesitis (i.e., the plantar aponeurosis, calcaneal entheses, and distal and proximal insertions of the patellar tendon), the tarsal joints, and the small toe joints is common in ERA. Definition of enthesitis includes abnormal hypoechoic (loss of normal fibrillar architecture) and/or thickened tendon or ligament as its bone attachment (may contain hyperechoic foci consistent with calcification) seen in two perpendicular planes that may exhibit a Doppler signal and/or bony changes such as enthesophytes, erosions, or irregularities [26]. Although enthesitis may be associated with bursitis, it must be noted that a minimal amount of bursal fluid can be detected with US in healthy children (Fig. 9.5). The examiner should be aware that enthesitis ultrasonographic image in children differs from adults mainly in the appearance of bony structure on which the tendon attaches. Depending on the child’s age, tendons and ligaments attach to layers of fibrocartilage and hypoechoic cartilage covering the secondary ossification center of the bone to which they insert. It is important to recognize the real tendon insertion site. For example, the distal portion of the patellar tendon lies in close apposition with the tibial epiphysis before inserting on the tuberosity. Additionally, when a growing child is examined by Doppler US, some vessels within the nonossified apophysis and epiphyseal cartilage can be detected indicating physiologic blood flow (Fig. 9.6; [37]).
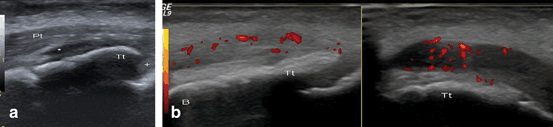
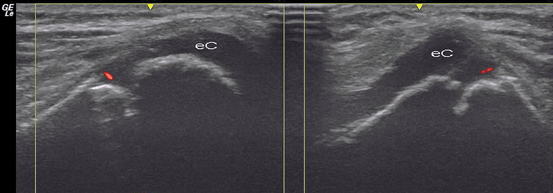

Fig. 9.5
Distal patellar tendinopathy. a Longitudinal B-mode US image shows distal insertion of the patellar tendon (Pt). Transducer should be positioned parallel to tendon insertion in order to assume a homogeneously hyperechoic appearance (avoiding anisotropy) . Deep to the patellar tendon, a minimal amount of bursal anechoic fluid can be observed (star). Note that under the deep infrapatellar bursa, a layer of hypoechoic fibrocartilage is covering the secondary ossification center of the tibial tuberosity (Tt) to which the patellar tendon inserts. Apophyseal cartilage (cross) of the tibial tuberosity remains between tibial diaphysis and secondary ossification center. b Longitudinal (left side) and transversal (right side) US images reveal a focal thickened and heterogeneous hypoechoic patellar tendon insertion with Power-Doppler signal reflecting active tendinopathy

Fig. 9.6
Healthy knee in a 6-year-old child. Longitudinal (left side) and transversal (right side) US images of the knee joint. Power Doppler US scan over the anterior-lateral surface of distal femur shows normal vascularization in epiphyseal cartilage (eC) of the femoral condyle. As the ossification process of the distal end of the femur has not been ended, the physis appears as cortical discontinuity in both images
US is able to show the ossification nuclei in the epiphyseal cartilage much earlier than when it becomes visible radiographically. However, US assessment of structural damage in children with JIA remains a challenge because of the peculiarities of the growing skeleton. Physis (or growth plate) showed as a discontinuity of cortical bone in the oldest children and physiological irregularities visualized by US in some ossification centers may be misinterpreted as erosions. Nevertheless, bony erosions can be detected by US with even higher sensitivity than that of conventional radiography [38]. As joint space narrowing is secondary to erosion and loss of articular cartilage , early US assessment showing decrease in joint cartilage thickness seems crucial in JIA [39]. On the other hand, epiphyseal vascularization can become conspicuous in inflammatory states of chronic disease (Fig. 9.7; [40, 41, 34]). Nevertheless, Doppler sensitivity to inflammatory flow (low-velocity flow) could depend partly on the settings and partly on the equipment.
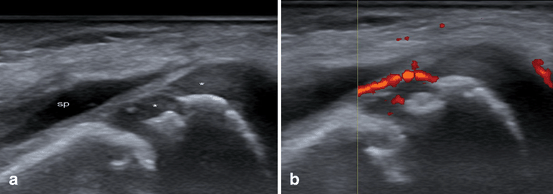

Fig. 9.7
Juvenile idiopathic arthritis in the knee joint. a Longitudinal B-mode US image shows the presence of suprapatellar bursitis (sp), erosion and decreased homogeneous hypoechogenicity of the femoral epiphyseal cartilage (stars). b Longitudinal Power Doppler US image pathologic vascularization involving the synovial tissue and epiphyseal cartilage close to the erosion. e secondary ossification center in epiphysis
US is the best available imaging technique for guiding needle position within inflamed joints and tendon sheaths in local procedures [34, 35]. Fluid aspiration for analyzing is important to exclude septic arthritis [42] or hemarthrosis in routine practice [43].
One of the most amazing issues for future searches is to identify the potential US role in the detection of subclinical disease in the pediatric population. US has recently been used to confirm the disease activity status and detect patients in disease remission. Earlier studies demonstrated that a significant number of JIA patients in clinical remission, have abnormal US findings, particularly in the wrist and ankle joints [44, 7]. A recent paper showed the superiority of US for detecting subclinical activity through the presence of positive PD signal in patients having inactive disease on and off medication [45]. The clinical relevance of those observations detected by US remains a critical issue that need to be addressed.
Septic Arthritis
Septic arthritis is infection of a joint which can occur either by direct spread of contiguous osteomyelitis, hematogenous dissemination from a remote site, or direct inoculation due to penetrating trauma. The most common presentation pattern of septic arthritis is monoarticular. In children younger than 3 years old, the hip is commonly affected, though the knee and ankle joints are also often involved. On the other hand, in neonates, due to the metaphyseal vascularization the association of osteomyelitis and septic arthritis is particularly more frequent. Clinical findings along with erythrocyte sedimentation rate greater than 40 mm/h, and white blood cell (WBC) count greater than 12,000/mm3 are strong predictors of an infected joint. Irritability and refusing to be breast fed are more common symptoms in neonates.
The main role of US in septic arthritis is the detection of joint effusion in a child with suspicion of infection. US is a handy tool for guiding pus joint aspiration [42]; It relieves intracapsular pressure, decreases damage to the articular surfaces, and helps to guide antibiotic treatment [46]. Although septic effusion often contains bright echoes and debris [47], US cannot be reliably used to differentiate septic arthritis from noninfectious arthritis based on the size and echogenicity of the effusion. On the other hand, an anechoic collection resulting from infected joint fluid is quite rare. Usually Doppler signal may be detected surrounding the joint at early disease (Fig. 9.8), although the absence of US features does not exclude it [48, 49].
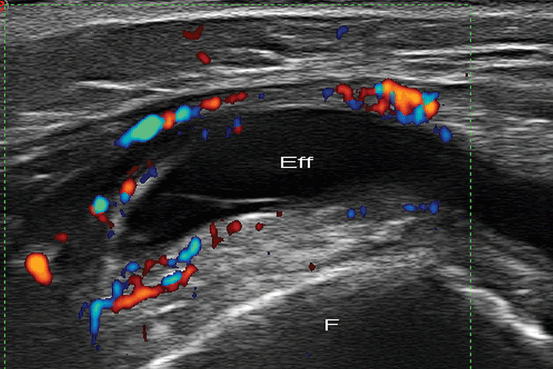

Fig. 9.8
Septic arthritis. Longitudinal color Doppler US image of the knee shows suprapatellar bursitis with synovial thickness and hyperemia reflecting acute arthritis
Transient Synovitis
In the field of pediatric rheumatology, US has been used in the initial workup of the hip disease in children having limited or no weight-bearing capability [50]. Transient synovitis (TS or toxic synovitis) represents the most common cause of acute nontraumatic painful hip in children between 5 and 10 years of age [51]. US allows early detection of effusion as the plausible cause. Managed with only supportive measures, such as avoidance of weight bearing and anti-inflammatory medication, it usually resolves in 3–10 days with no long-term consequences. US is also used for monitoring and follow-up of children with longer or repeated episodes of TS in order to rule out early Perthes disease.
The differential diagnosis of TS includes infection (septic arthritis, osteomyelitis [52], Legg–Calvé–Perthes disease, slipped capital femoral epiphysis (SCFE), JIA, or tumors [53]).
In TS, effusion is identified as the only cause of distention of the anterior joint recess; no significant thickening of both layers of the capsule exists (Fig. 9.9). The anterior-sagittal approach clearly demonstrates joint effusion within the anterior recess of the hip capsule. The joint capsule is normally concave anteriorly and close to the femoral neck. A minimum of about 1 mm3 of fluid may be detected intracapsular, allowing differentiation of both layers of the capsule [54]. On B-mode ultrasonographic image, an effusion is diagnosed when the joint capsule bows anteriorly in addition to: (1) distension more than 5.2 mm with fluid measured from the middle of the femoral neck to the capsular outer margin [28]; or (2) when the distension of the capsule is 2 mm or more than the contralateral asymptomatic side. Often the asymptomatic contralateral joint offers the best standard for comparison. Hip aspiration should be recommended to patients who pose a diagnostic problem, such as a high level of suspicion for septic arthritis or a chronic rheumatologic disorder [42, 55]. Unlike JIA and infectious diseases , Doppler signal is not visualized in the hip capsule.
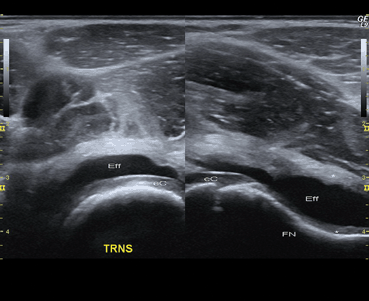

Fig. 9.9
Transient synovitis. Transverse and longitudinal B-mode US images of the hip joint show distended anterior recess due to the presence of anechoic effusion (Eff) without thickening of the hip capsule layers associated (stars). The surface of the hypoechoic cartilage of the femoral head is clearly visible as a strong reflection (interference sign) between the effusion and the epiphyseal cartilage (eC)
Legg–Perthes–Calvé Disease
Legg–Perthes–Calvé disease (LPCD) is an idiopathic osteonecrosis of the immature capital femoral epiphysis. It occurs in children between 2 and 14 years of age, with a peak incidence at 5–6 years old, male predominance, and mostly unilateral involvement. Although the causes of LPCD disease are unknown, there is considerable evidence that the disease might have an underlying vascular mechanism [56, 57]. LPCD may also occur as a result of recurrent TS in patients who have long-lasting effusion and symptoms [58]. Clinical symptoms include limp and pain in the hip, thigh, or knee.
In early LPCD, the radiography may be normal or show collapse of the femoral head [59]. Though there are no specific US features, hip effusion can be visualized in early disease. The permanence of effusion or irregular echogenicity may help to suspect the disease. After the ischemic phase, US can show irregularities in femoral head contour because of fragmentation of epiphysis, and deformity ([60]; Fig. 9.10). Using Doppler technique, Doria [57] has described some changes in the intraosseous/deep transphyseal vascularity of the proximal femur in children with LPCD.