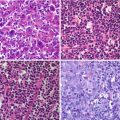
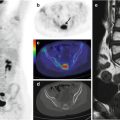
Fig. 21.1
A 5-year-old girl with Rasmussen’s encephalitis and partial seizures since the age of 3 years. (a) Axial PET image, (b) PET/MRI fusion. FDG was fortuitously administered during a seizure and shows, with very high spatial resolution and excellent image contrast, the involved cortex in the left hemisphere
21.2 FDG–PET in Temporal Lobe Epilepsy
The value of FDG–PET has been best proven in the evaluation of medically refractory epilepsy patients who are candidates for surgery, specifically those with clinically suspected temporal lobe epilepsy. In this setting, the sensitivity of FDG–PET is between 80 and 90 % [1–4]. Only a few studies have addressed the sensitivity and specificity of FDG–PET in medial temporal lobe epilepsy patients with and without evidence of hippocampal sclerosis on MRI. However, FDG–PET is still considered to be reliable in lateralizing the epileptogenic temporal lobe even in MRI-negative patients, as shown in Fig. 21.2 [5]. Of course, MRI techniques are constantly evolving, and new sequences and new analytical approaches may one day allow the identification of focal alterations in cases previously considered to be “MRI negative.”
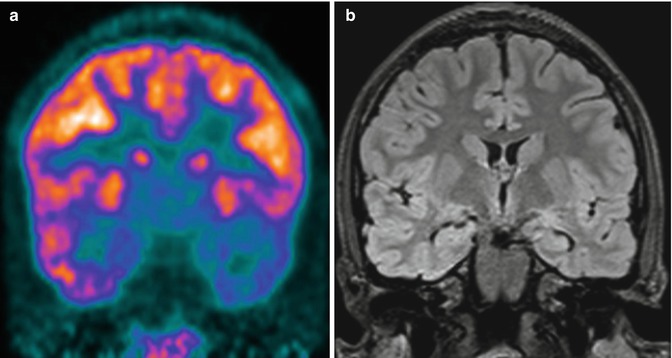

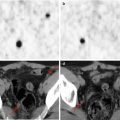
Fig. 21.2
A 13-year-old boy with partial complex epileptic seizures. (a) Coronal PET image, (b) MRI image. MRI does not show any clear abnormality, while on the PET image, there is marked hypometabolism in the left temporal pole. A repeated MRI investigation identified an area of probable cortical dysplasia. A left anterior temporal lobectomy was planned
21.3 FDG–PET in Extratemporal Epilepsy
The clinical value of FDG–PET in neocortical epilepsy is less clear. The larger reported series consist of observational retrospective studies, and only a few were performed in the era of advanced MRI techniques [1, 6]. Most importantly, the FDG–PET findings in nonlesional neocortical epilepsy are often obtained from heterogeneous patients and patient groups. An example of the FDG pattern in a specific syndrome such as tuberous sclerosis is provided in Fig. 21.3 [7].
In tuberous sclerosis typically FDG-PET shows hypometabolism in and around tubers, believed to be due to decreased neuronal number and simplified dendritic pattern. A tuber with a disproportionately large area of hypometabolism compared with its size on MR images is most likely epileptogenic [8]. However, both epileptic and nonepileptic tubers show reduced uptake on FDG-PET, while promising results have been obtained using a tracer specific for the serotonergic system, the α-[11C]methyl-l-tryptophan (AMT). AMT-PET shows increased AMT uptake interictally in epileptic but not quiescent tubers in almost two-thirds of children with tuberous sclerosis and intractable epilepsy: all tubers with at least 10% AMT increase were found to be epileptogenic [11].
Co-registered multimodality imaging may provide other supportive localizing information confirming that a questionable PET metabolic abnormality is indeed a true disturbance reflective of the epileptogenic zone, thus emphasizing the added value of imaging fusion or hybrid imaging, when available [9, 13, 14].
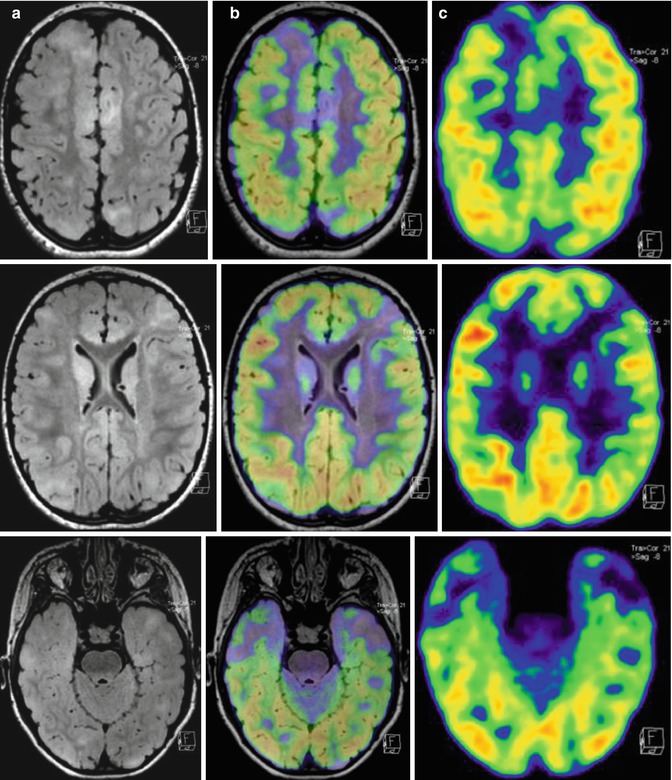

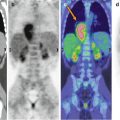
Fig. 21.3




A 5-year-old girl who at the age of 5 months was diagnosed with tuberous sclerosis and generalized epileptic seizures. (a) Axial MRI, (b) PET/MRI fusion, and (c) PET images. The PET/MRI fusion images show multiple cortical lesions in the two hemispheres, typically hypometabolic on FDG–PET imaging. However, these imaging modalities do not allow localization of the lesion generating the epileptic seizures. Instead, promising results were obtained using α-[11C]methyl-l-tryptophan (AMT), a tracer specific for the serotonergic system
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree