Fig. 1
[68Ga]-DOTATOC study of a patient with neuroendocrine tumor. From left to right axial images of: a attenuation-corrected PET, b CT, and c PET/CT. Without any anatomical correlated uptake, highly specific PET tracer uptake cannot be localized sufficiently; here, combined imaging becomes mandatory. Data courtesy of Hofmann (Hannover) and Bockisch (Essen)
However, despite the success and wide distribution of PET/CT, there are some shortcomings in use of CT as the anatomical complement to PET. CT uses a source of ionizing radiation for imaging and, therefore, adds significant radiation dose to the overall examination (Brix et al. 2005), which may raise concern in selected populations such as adolescents, females, and patients with benign diseases. Further, CT provides comparatively low soft tissue contrast unless additional CT contrast agent are used. MR, on the other hand, does not suffer from these two major disadvantages and, in addition, offers more advanced functional imaging information, such as dynamic contrast-enhanced (DCE), diffusion-weighted imaging (DWI), or magnetic resonance spectroscopy (MRS), without adding to the overall radiation exposure burden.
The combination of PET and MR in a single imaging system has the potential to become a multimodality imaging technology of choice combining anatomical, functional, and molecular information into a single multiparametric imaging modality. Nonetheless, it is hard to make hypotheses on the main clinical applications of combined PET/MR at this stage (Beyer and Pichler 2009), where first prototype systems are being validated in clinical and research settings (Pichler et al. 2010). The potential areas of application of combined PET/MR imaging in brain imaging, for example, benefit greatly from the additional morphologic information provided by MRI. Combined amino acid PET and MR imaging is likely to enhance diagnostic sensitivity for gliomas and may allow closer correlation between tracer uptake and metabolic changes (e.g., choline peaks in MR spectroscopy) in neoplastic tissue (Bisdas et al. 2009). Likewise, arterial spin-labeling estimations of perfusion and diffusion changes occurring in low-grade gliomas may be studied in conjunction with different PET tracers to establish reliable disease markers. Boss and colleagues recently evaluated simultaneous PET/MRI for assessing intracranial tumors using [11C]-methionine for glioma or [68Ga]-DOTATOC for meningioma. They demonstrated image quality and quantitative data achieved from PET/MRI to be similar to that of PET/CT (Boss et al. 2010).
Given the availability of combined PET/MR systems, functional MRI and molecular PET image data can be acquired simultaneously, thus offering much improved spatial and temporal co-registration, which may become beneficial for image-guided radiotherapy (RT) treatment planning of various oncological diseases (Werner et al. 2011).
2 Methods
2.1 PET/MR Tomography
MR requires very high field homogeneity. Furthermore, the presence of PET detectors within the field could interfere with MR imaging. In contrast, PET detectors have to withstand not only a high static field level (up to 3 T for clinical MR) but also the rapidly changing field gradients required by the imaging process. PET/MR was destined to remain in the preclinical arena for another decade until, in 2006, the first simultaneous MR and PET images of the human brain were acquired (Schmand et al. 2007).
Existing hardware concepts for clinical PET/MR imaging essentially fall into three categories (Fig. 2): (a) separate gantries operated in different rooms, (b) gantries arranged in the direction of the main scanner axis with a patient-handling system mounted in between, and (c) fully integrated systems. The first design, presented in 2006 and also being the most challenging (Fig. 2c), is based on a PET detector ring designed as an insert that can be placed inside a Siemens 3-T Trio MR scanner (Siemens Healthcare). This prototype system (BrainPET) was anticipated for brain imaging only (Schlemmer et al. 2008). A similar design was replicated and made available for whole-body applications in late 2010 by the same company.
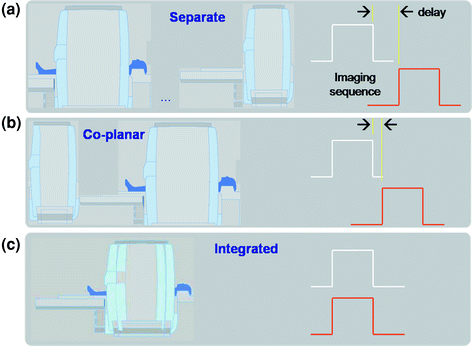

Fig. 2
System designs for combined PET/MR tomography: a PET (or PET/CT) and MR are situated in separate rooms and combined via a mobile, docking table platform, b PET and MR are within the same room with a joint table platform, and c integrated design where the PET component fits within the MRI gantry, and data are acquired simultaneously
The coplanar PET/MR concept (Fig. 2b), first presented in 2010, is based on a tandem design of a whole-body time-of-flight (TOF) (Budinger 1983) PET system and a 3-T Philips Achieva MR system (Philips Healthcare, Cleveland, USA) with a rotating table platform in between. Through minor modifications of the PET detector system (e.g., orientation of the photomultiplier tube (PMT), minor shielding) the PET gantry can be operated in close proximity to the 3-T MRI system.
The third design was proposed by GE Healthcare in late 2010 and is available as prototype technology only. This design is based on a combination of a dual-modality PET/CT and a 3-T MR system that are operated side-by-side in separate rooms next to each other; patients are shuttled from one system to the other without getting off the bed. This approach substitutes the challenges of hardware integration for immense logistical challenges in timing access to the two systems while minimizing patient motion in between examinations. However, this approach has been argued as the most cost-effective compared with fully integrated PET/MR based on workflow aspects and machine utilization (Schulthess and Burger 2010).
In addition to the technical challenges of combining PET and MRI, which increase with the amount of PET and MR system integration, the necessary attenuation correction factors for the PET emission data must be derived from PET/MR measurements (Hofmann et al. 2009). While in PET/CT PET attenuation data can be derived from transforming available CT transmission images into maps of attenuation coefficients at 511 keV (Kinahan et al. 2003), no such transmission data are available in PET/MR. This is primarily due to the lack of physical space to host a transmission source. Therefore, PET/MR requires novel approaches to MR-based attenuation correction (MR-AC). While segmentation-based approaches have been proposed to classify tissues on MR images and to assign respective attenuation coefficients, which work well in brain imaging (Zaidi 2007); MR-AC in extracerebral applications is, however, much more challenging (Beyer et al. 2008).
2.2 Diagnosis and RT Treatment Planning of Meningioma
Meningiomas are mesodermal tumors originating from the arachnoid membrane and represent one of the most frequently diagnosed types of brain tumor. Incidence is about 25 per million people, peaking in patients between 40 and 70 years and increasing with age. Henze et al. (2001) demonstrated for the first time that PET imaging using [68Ga]-DOTATOC [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-d-Phe1-Tyr3-octreotide (TOC)] is a promising diagnostic test for meningioma patients. This is due to the fact that meningiomas are known to express somatostatin receptor subtype 2 (SSTR2) for which, consequently, [68Ga]-DOTATOC can be used as a biomarker to visualize the extension of meningiomas in PET or PET/CT studies. Henze et al. (2001) showed good correlation of tracer boundaries with anatomical delineation obtained from (co-registered) CT and MR image volumes, even for small meningiomas of 7 mm.
Treatment of meningiomas can be difficult due to their proximity to organs at risk (e.g., brainstem, cranial nerves). Although surgical resection is the main treatment approach, RT has been reported to be a highly effective treatment modality (Milker-Zabel et al. 2007). Until now, RT treatment planning has been based mainly on CT and contrast-enhanced MRI (Debus et al. 2001). However, in the case of quasiplanar growth, these imaging techniques have limitations concerning visualization of the exact tumor extension. Thus, PET or PET/CT is highly valuable as an additional imaging modality for more accurate target volume (TV) delineation (Milker-Zabel et al. 2006).
Intensity-modulated radiation therapy (IMRT) is an effective technique for treating meningiomas with high levels of conformality. A combination of CT, MR, and [68Ga]-DOTATOC-PET imaging has been reported to improve TV delineation for IMRT treatment planning (Gehler et al. 2009; Thorwarth et al. 2011).
2.3 68Ga-DOTATOC PET/MR Protocol
Here we present three cases of patients with meningiomas undergoing [68Ga]-DOTATOC PET/MR imaging. All patients had a clinical indication for a PET/CT study, which was performed prior to the PET/MR study.
The PET/CT examination was performed with a Biograph HiRez 16 unit (Siemens Healthcare, Knoxville, USA), which is equipped with 4 × 4 × 16 mm3 lutetium oxyorthosilicate (LSO) scintillation crystals in combination with a 16-slice CT. Patients were injected intravenously with an average activity of 150 MBq of [68Ga]-DOTATOC. Static emission scans of two overlapping bed positions in the tumor region were acquired 40 min post injection (p.i.), with acquisition time of 4 min per bed position. PET images were reconstructed iteratively using a two-dimensional (2D) ordered-subset expectation-maximization (OSEM) algorithm with 4 iterations and 8 subsets. CT-based attenuation and scatter correction was performed. Reconstructed PET data had in-plane resolution of 6 mm and slice thickness of 5 mm.
PET/MR imaging commenced immediately after the completion of the PET/CT acquisition in a different part of the hospital. A prototype PET/MR system dedicated to brain imaging (BrainPET; Siemens Healthcare, Knoxville, USA) was used (Schmand et al. 2007). Briefly, a clinical 3-T MR system (Magnetom Trio; Siemens Healthcare, Erlangen, Germany) is equipped with an MR-compatible brain PET insert. The PET detector ring consists of 2.5 × 2.5 × 20 mm3 LSO scintillation crystals in combination with avalanche photodiodes (APD). The PET resolution is less than 3 mm in the center of the field of view. PET data acquisition started approximately 100 min p.i. List-mode data were acquired over a period of 30 min to compensate for the longer uptake time. PET reconstruction was performed using a 3D ordinary Poisson OSEM resolution-modeling algorithm with 6 iterations and 16 subsets, 3D scatter correction, and MRI-based attenuation correction (Hofmann et al. 2008).
MRI was performed using the birdcage transmit/receive coil, which is suitable for all routine clinical examinations. The MR sequences used for examinations of the brain with combined PET/MR are described in detail elsewhere (Boss et al. 2010). The combined PET/MR machine allows PET and MR acquisition of data not only in the same geometrical localization but also simultaneously.
2.4 Radiotherapy Treatment Planning
For all patients, IMRT treatment planning was performed using 6 MV photons from seven different beam angles. IMRT was planned with a dynamic delivery technique using a linear accelerator equipped with a multileaf collimator with leaf widths of 4 mm in the isocenter plane (Elekta Synergy S, Crawley, UK). For accurate patient positioning, a thermoplastic mask system was used in addition to onboard position verification with the cone-beam CT system.
First, IMRT TVs were delineated manually based on a combination of CT, [68Ga]-DOTATOC PET, and separately acquired MR data (Gehler et al. 2009). A second set of contours was created using only combined PET/MR imaging data in addition to the planning CT required for RT dose calculation. IMRT treatment planning was performed using the PET/CT-based TVs as reference, which was then cross-evaluated for the PET/MR-based TVs to evaluate the dosimetric differences imposed by PET/MR-guided IMRT planning.
2.5 Clinical Examples
Case 1 A 73-year-old female presented with recurrence of an atypical meningioma (WHO II) in the right anterior central region 15 months after complete resection. Clinical findings before first resection were left-sided gait abnormality and disease progression on MR imaging. Postoperative imaging consisting of MRI and [68Ga]-DOTATOC-PET/CT did not determine any residual tumor. Due to aggravation of the pre-existing gait abnormality 15 months after surgery, another set of MRI and [68Ga]-DOTATOC-PET/CT examinations was performed, and recurrent disease was detected. This case was discussed in an interdisciplinary neurooncology tumor board, and radiation treatment was recommended.
First, TV delineation was performed separately on PET/CT and MR image volumes. Fusion of these two TVs resulted in a total gross target volume (GTVPET/CT+MR) of 12.7 mL. In a second step, a corresponding GTVPET/MR was defined exclusively based on simultaneous PET/MR, which had size of 13.5 mL. Subsequently, both GTVs were expanded isotropically by 4 mm to create the respective planning target volumes (PTVs). Figure 3 shows the TVs defined on the basis of PET/CT and MR (PTVPET/CT+MR and GTVPET/CT+MR) (Fig. 3b) and PET/MR (PTVPET/MR and GTVPET/MR) (Fig. 3d) superimposed on the planning CT. PET/CT- and PET/MR-based TV delineation resulted in PTVs of 28.0 and 31.5 mL, respectively.
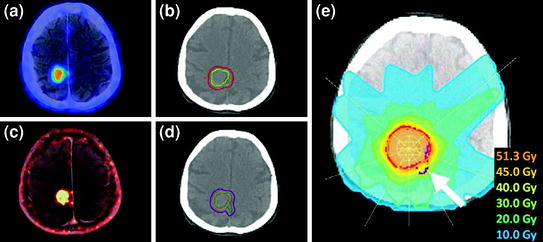

Fig. 3




Patient 1: 73-year-old female with meningioma WHO grade II following [68Ga]-DOTATOC PET/CT and PET/MR: axial PET/CT (a) and PET/MR image (c), GTVs based on PET/CT (yellow) and additional MR (green) data in addition to the resulting PTV (red) (b), and after TV delineation based solely on hybrid PET/MR (PET: green, simultaneously acquired MR: orange, PTV: purple) (d). IMRT plan created for TVs based on PET/CT only (red) and cross-evaluated for TVs delineated on simultaneous PET/MR image data (purple) shown in (d). The arrow shows an infiltrated tumor region only visualized on hybrid PET/MR, which would have been significantly underdosed after TV delineated based solely on combined PET/CT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree