Chapter 8 Pleural Tumors
Introduction
MPM is an uncommon neoplasm arising from mesothelial cells of the pleura. The annual incidence is 3000 cases in the United States. The worldwide figure is expected to increase in the coming decade owing to the patterns of occupational exposure to asbestos and latency period of up to 50 years.1 There is currently no universally accepted standard therapy for MPM and the prognosis is poor, with a median survival of 9 to 17 months after diagnosis.2 However, important advances in the treatment of patients with MPM have occurred over the past few years, including a unified staging system, novel targeted agents, improved radiation therapy techniques for local control, and decreased morbidity and mortality in patients who undergo curative surgical resection.1,3 Furthermore, multimodality regimens combining chemotherapy, radiotherapy, and surgery are being used more frequently because of the failure of single-modality therapy. In cases of limited disease, there has been an increasing tendency to perform surgical resection as part of the treatment algorithm. Extrapleural pneumonectomy (EPP), the removal of the visceral and parietal pleura, ipsilateral lung, hemidiaphragm, and part of the pericardium, is the surgical treatment of choice in the 10% to 15% of patients who present with resectable disease and is reported to prolong survival (74% 2-year survival and 39% 5-year survival).4 The greatest survival benefit in patients with MPM after EPP is seen in those with epithelial histology, a primary tumor that is limited in extent, and no nodal metastases. Conversely, patients with sarcomatoid histology and nodal metastases have a poor survival benefit after EPP and are typically primarily treated with palliative chemotherapy.5
Epidemiology and Risk Factors
MPM occurs more frequently in men than in women with a ratio of 4:1; however, the incidence in women is increasing.6 Peak incidence occurs in the sixth to seventh decades of life and is associated with a history of occupational exposure to asbestos in 40% to 80% of patients.7 In asbestos workers, the incidence of MPM is 10%.8 In contrast, the incidence of MPM in the general population is lower, estimated at 0.01% to 0.24%.7,8
Asbestos, a collective term for a group of complex hydrated silicates, has varying degrees of carcinogenicity. MPM develops after a latent period of up to 50 years from exposure to asbestos. There are two principal forms of asbestos: long, thin fibers known as amphiboles (amosite and crocidolite) and serpentine fibers known as chrysotile. The risk is low if exposed to chrysotile asbestos only. There is a dose-response relationship between crocidolite asbestos exposure and MPM. The exposure-specific risk of MPM from the three principal commercial asbestos types is approximately 1:100:500 for chrysotile, amosite, and crocidolite.9 Chrysotile accounts for approximately 80% of the asbestos used in the Western world. Occupations at highest risk include insulation work, asbestos production and manufacture, heating industry, shipyard work, construction, and automotive brake-lining manufacture and repair.7 Based on regulation by environmental regulatory bodies and the latency period, predictions for a MPM peak in the United States was 2004; Australia, 2015; Europe, 2020; and Japan, 2025.10 However, these predictions did not include many unanticipated factors such as the World Trade Center attack in 2001 in which an estimated 10 million New Yorkers were exposed to asbestos dust.11,12
Because 20% of MPM patients do not have an exposure to asbestos, alternative factors are presumed to be involved. Simian virus 40 (SV40), a DNA virus, has been implicated as a cofactor in the cause of MPM. SV40 nucleic acids have been documented in a proportion of MPM cases.13 This virus blocks tumor suppressor genes and is a potent oncogenic virus in human and rodent cells.
Molecular biologic features of angiogenesis in MPM are important in the development of novel therapeutic strategies. MPM cells produce many growth factors such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF08-β).14–16 MPM expresses the highest known levels of vascular endothelial growth factor (VEGF) of any solid tumors. VEGF expression in MPM is associated with poor survival and is now considered to be an independent prognostic factor in MPM. There is a positive correlation between VEGF expression and tumor stage (P < .05).17 VEGF inhibitors have been shown to reduce MPM growth in animal models. Studies on the use of antiangiogenesis agents to target the VEGF pathway are ongoing. These agents include PTK787, an inhibitor of the PDGF/VEGF pathway, and bevacizumab, a recombinant human anti-VEGF monoclonal antibody.16 Genetic alterations in tumor-suppressor genes such as p16, p14, and NF2 are common, and the activity of the antiapoptosis molecule Bcl-xL is elevated in MPM.12,18 Furthermore, MPM cells usually express telomerase, enabling cells to be resistant. In addition, interleukin-8, a potent chemokine with proangiogenesis function, has been shown to be an autocrine growth factor in MPM cell lines.16
Anatomy and Pathology
The anatomy of the pleura is complex. The inferior margins of the pleura in the posterior costodiaphragmatic recesses of the hemithorax extend considerably lower than the corresponding border of the lung, to the level of the T12 vertebra. The diaphragm extends more inferiorly and arises from the anterolateral surfaces of the upper three lumbar vertebrae. Macroscopically, the affected lung is covered by a thick layer of soft, gelatinous, grayish-pink tumor. Microscopically, MPM is classified into three histologic categories that provide a foundation for prognosis and therapy, forming a critical basis for epidemiologic and clinical studies. These categories are epithelial (55-65%), sarcomatoid (10-15%), and mixed or biphasic (20-35%).19 The desmoplastic variant is considered a subtype of sarcomatous diffuse MPM. The epithelial type consists of cuboidal or polygonal cells with abundant pink cytoplasm and uniform round nuclei forming a tubular and papillary structure. The sarcomatoid or mesenchymal type of MPM consists of sheets of spindle cells of variable size, cellularity, and pleomorphism. The mixed type of MPM contains both epithelial and sarcomatoid patterns. The World Health Organization (WHO) classification requires that 10% or more of each component be present to fit the classification of biphasic.20 Special features of MPM include positive staining for acid mucopolysaccharide, strong staining for keratin proteins, and on electron microscopy, the presence of long microvilli and abundant tonofilaments but absence of microvillous rootlets and lamellar bodies. To differentiate epithelial MPM from adenocarcinoma, immunohistochemistry panels are useful. Epithelial MPM cells are positive for certain keratin proteins (AE1/AE3, CK5/6, CK7), calretinin, WT-1, D2-40, HBMe1, mesothelin, and thrombomodulin and negative for many markers including pCEA, TTF1, CD15(Leu-M1), BerEp4, B72.3, BG-8, and MOC-31.20 In contrast, immunohistochemistry is less helpful in sarcomatoid diffuse MPM.
Cytologic evaluation of pleural fluid (26% sensitivity) and needle aspiration biopsy (20.7% sensitivity) are inadequate to diagnose MPM.21 If tumor cells are present, distinguishing MPM from metastatic adenocarcinoma or severe atypia can be difficult. In contrast, image-guided core needle biopsy to obtain larger tissue samples has been shown to improve diagnostic accuracy (77% with ultrasound guidance and 83% with CT guidance).22 When a larger diagnostic specimen is needed, a Cope needle biopsy, video-assisted thoracoscopic surgery (VATS), or open biopsy is performed. VATS has a diagnostic rate of 98% and is becoming the preferred method of diagnosis. However, this procedure has two disadvantages: the visceral and parietal layers of the pleura must not be adherent and chest wall seeding occurs in up to one half of the patients.6,21 In contrast, chest wall seeding occurs in 22% of image-guided biopsies.22 To prevent tumor growth within biopsy sites, trocar ports, thoracoscopic tracts, and chest tube tracts, patients undergoing EPP typically have these tracts resected. In addition, local radiation therapy can be used to prevent chest wall seeding.
Key Points Anatomy and pathology
• MPM involves parietal and visceral pleural surfaces and extends into the interlobar fissures, along the diaphragm, mediastinum, and pericardium.
• Tumor can invade lung and peritoneum.
• MPM is divided into three histologic categories: epithelial (55-65%), sarcomatoid (10-15%), and mixed or biphasic (20-35%).
Patterns of Tumor Spread
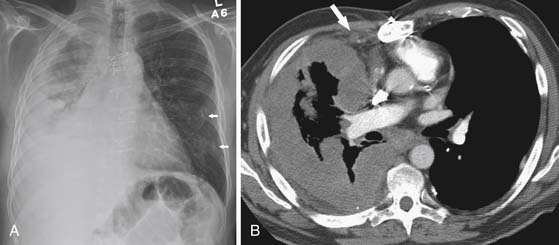
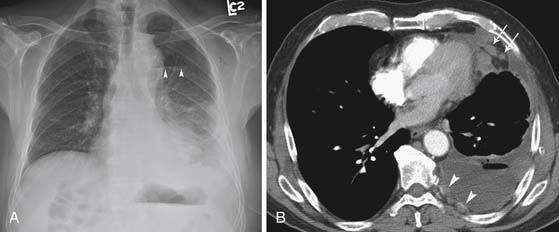
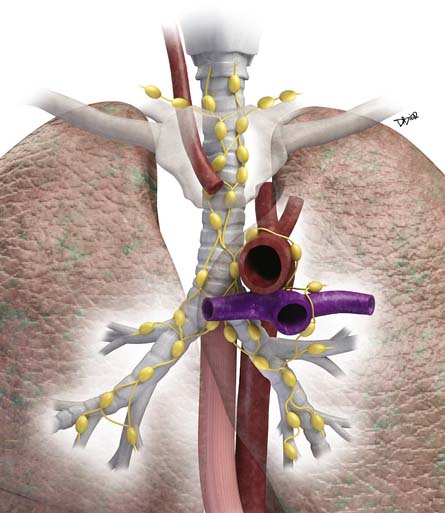
Lymphatic dissemination is common and mediastinal nodes are involved in 50% of cases. To understand the lymphatic spread of MPM, it is essential to examine the complex lymphatic drainage system of the pleura. The visceral pleural lymphatics follow the same drainage pattern as the lungs. However, the parietal pleural lymphatic drainage system is different. The anterior parietal pleura drains into the internal mammary lymph nodes (Figure 8-1). The posterior parietal pleura drains into the extrapleural/intercostal lymph nodes, which are located in the paraspinal fat adjacent to the heads of the ribs (Figure 8-2). The anterior and lateral diaphragmatic lymphatics drain into the internal mammary and anterior diaphragmatic lymph nodes. The posterior diaphragm drains into the para-aortic and posterior mediastinal lymph nodes. There are free anastomoses between lymphatics on both surfaces of the diaphragm, including the retrocrural, inferior phrenic and gastrohepatic space, and the region of the celiac axis.
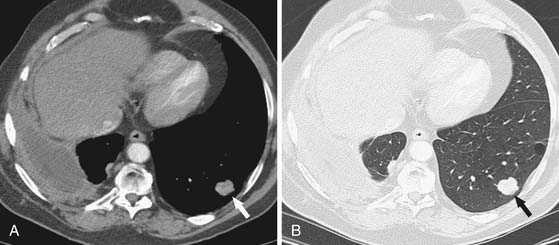
Distant hematogeneous metastases are common and can involve the lungs, liver, spleen, adrenals, lymph nodes, bones, and brain (Figure 8-3). Extrathoracic metastatic disease has been documented at autopsy in 50% to 80% of cases.23
Key Points Tumor spread
• Local spread involves the parietal and visceral pleura, extends to interlobar fissures, and along the diaphragm, mediastinum, and pericardium.
• Owing to the complex drainage system of the pleura, evaluation of nodal disease in the extrapleural/intercostal, internal mammary, diaphragmatic, and upper abdominal regions is essential.
• Mediastinal nodal disease is seen in 50% of cases.
• Transdiaphragmatic invasion can result in spread to the peritoneum, liver, and spleen.
• Hematogeneous dissemination occurs in 50% to 80% at autopsy.
Staging Evaluation
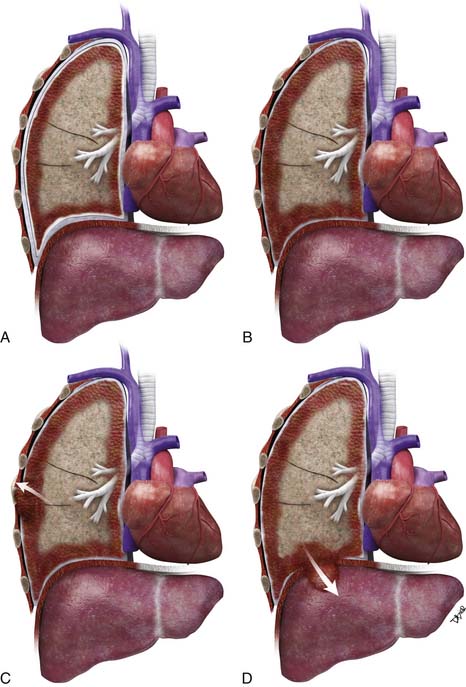
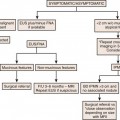
Multiple staging systems have been proposed for MPM.24,25 In an attempt to distinguish patients who would benefit from surgical resection from those needing palliative treatment, the International Mesothelioma Interest Group (IMIG) staging system for MPM was proposed and is gaining universal acceptance (Tables 8-1 and 8-2).26 This system describes the extent of tumor according to a traditional tumor-node-metastasis (TNM) classification: local extent of the primary tumor (T descriptor), the presence and location of lymph node involvement (N descriptor), and the presence or absence of distant metastatic disease (M descriptor) (Figures 8-4 and 8-5). This system stratifies patients into categories with similar prognoses in an effort to select homogeneous groups of patients for entry into clinical trials to better assess new treatment options. Primarily to identify patients who are potentially resectable, this staging system uses criteria to determine the extent of local tumor and regional lymph node status, two factors that have been shown to be related to overall survival rate.26,27 The presence of advanced locoregional primary tumor (T4), N2-N3 disease (mediastinal, internal mammary, and supraclavicular lymph nodes), and M1 disease preclude surgery. However, staging using imaging modalities such as CT, MRI, and PET has limitations. This limitation together with the morbidity and mortality associated with EPP has resulted in the need for extended surgical staging (ESS) in patients being evaluated for resection. In our institution, cervical mediastinoscopy or endobronchial ultrasound-guided lymph node biopsy, laparoscopy, and peritoneal lavage are routinely performed in MPM patients undergoing preoperative evaluation. Rice and coworkers28 reported that ESS precluded 15 of the 118 patients (12.7%) assessed by clinical staging alone to be candidates for EPP.
Table 8-1 Tumor-Node-Metastasis International Staging System for Diffuse Malignant Pleural Mesothelioma
| T—Primary Tumor | |
| T1a | Tumor limited to ipsilateral parietal pleural, including mediastinal and diaphragmatic pleura |
| No involvement of visceral pleura | |
| T1b | Tumor involving ipsilateral parietal pleura, including mediastinal and diaphragmatic pleura |
| Scattered foci of tumor also involving visceral pleura | |
| T2 | Tumor involving each ipsilateral pleural surface with at least one of the following features: |
| T3 | Locally advanced but potentially resectable tumor Tumor involving all of ipsilateral pleural surfaces with at least one of the following: |
| T4 | Locally advanced technically unresectable tumor Tumor involving all of ipsilateral pleural surfaces with at least one of the following: • Diffuse extension or multifocal masses of tumor in chest wall, with or without associated rib destruction • Direct transdiaphragmatic extension of tumor to peritoneum • Direct extension of tumor to contralateral pleura • Direct extension of tumor to one or more mediastinal organs • Direct extension of tumor into spine • Tumor extending through to internal surface of pericardium with or without pericardial effusion or tumor involving myocardium |
| N—Lymph Nodes | |
| NX | Regional lymph nodes not assessable |
| N0 | No regional lymph node metastases |
| N1 | Metastases in ipsilateral bronchopulmonary or hilar lymph nodes |
| N2 | Metastases in subcarinal or ipsilateral mediastinal lymph nodes, including ipsilateral internal mammary nodes |
| N3 | Metastases in contralateral mediastinal, contralateral internal mammary, and ipsilateral or contralateral supraclavicular lymph nodes |
| M—Metastases | |
| MX | Distant metastases not assessable |
| M0 | No distant metastases |
| M1 | Distant metastases present |
Table 8-2 Staging Classification of Stage by Tumor-Node-Metastasis Description
| STAGE | DESCRIPTION |
|---|---|
| Ia | T1aN0M0 |
| Ib | T1bN0M0 |
| II | T2N0M0 |
| III | Any T3M0 |
| Any N1M0 Any N2M0 | |
| IV | Any T4 |
| Any N3 Any M1 |
It is important to note that imaging is inaccurate in determining the true extent of MPM. When compared with surgical staging, CT has been shown to underestimate the extent of disease in patients with early chest wall involvement, small positive lymph nodes, transdiaphragmatic extension, peritoneal implants, and abdominal organ metastases less than 2 mm in size.29 Despite these limitations, CT with its easy accessibility and cost-effectiveness remains the imaging modality of choice in the initial staging and follow-up of patients with MPM.
T Staging
Accurate T staging is emphasized by the IMIG primarily to determine resectability.26 In patients with locally advanced tumors, radiologic imaging is usually directed at distinguishing T3 disease (a solitary focus of chest wall involvement, involvement of the endothoracic fascia, mediastinal fat extension, or nontransmural pericardial involvement) from nonresectable (T4) disease (diffuse tumor extension or multiple chest wall foci; direct extension to the mediastinal organs, spine, internal pericardial surface, or contralateral pleura; and transdiaphragmatic invasion) (Figure 8-6). However, the parameters for T staging are pathologic descriptors that are often difficult to determine by CT and MRI.
In locally advanced (T4) disease, the poor accuracy of older CT in assessing transdiaphragmatic extension of MPM is due to its inability to detect small volume and microscopic invasion as well as the inherent limitation of axial imaging to delineate the diaphragm from the primary pleural tumor. With the use of multidetector row computed tomography (MDCT), PET/CT imaging allows high-resolution multiplanar reconstruction to better evaluate the diaphragm. However, the accuracy of PET/CT is also suboptimal in detecting subtle transdiaphragmatic extension. Because of the limitation of imaging, preoperative laparoscopy is routinely performed in our institution in patients being evaluated for EPP. In the study by Rice and coworkers,28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree