The x-ray generator allows for the selection of kilovolt peak (kVp) and tube current (mA) that is delivered to the x-ray tube. Generator types may be single phase, three phase, constant potential, and high frequency. Continuous or pulsed exposure is used to energize the tube. The main advantage of pulsed exposure is reduction in radiation exposure. There may be improvement in temporal resolution and decreased motion artifact. High-frequency generators provide superior exposure reproducibility and are the most compact and the cheapest, making them the most commonly used equipment [4].
The x-ray tube converts electrical energy produced by the generator into an x-ray beam.
The collimator defines the shape of the x-ray beam and limits the x-ray beam to the field of vision. The x-ray beam should be collimated to the area of interest to decrease the radiation to the tissue, therefore reducing the scatter produced and improving image contrast. When using collimation, it is important that the electronic collimation matches the manual collimation. Electronic collimation can mask critical information included on the image and cover up anatomy that is exposed [5].
Filters are used to attenuate low energy x-rays from the beam, which are absorbed by the patient but not transmitted to the image receptor. By absorbing these rays, filters aid in decreasing exposure to the patient.
Anti-scatter grids reduce the scattered x-rays reaching the image receptor, thereby improving the image contrast. However, this requires an increase in radiation exposure. Grids should be removed when performing studies where the scatter is low, particularly in pediatrics.
The image intensifier converts x-rays into a visible light image and amplifies the image brightness for better visibility [4]. Optical coupling distributes light from the image intensifier output window to a video camera and other image recording devices. A television system is used to view the image and a recording device stores the images.
Radiation Exposure and Radiation Safety
When using radiation, the ALARA principle, or a s l ow a s r easonably a chievable, should be implemented. This term was first used to refer to power plant and other radiation workers and has subsequently been applied to the medical imaging. Decreasing radiation exposure is especially important when children are involved as the radiation effects are cumulative, lifelong, and children are more sensitive to the effects of radiation. The linear no-threshold model of potential radiation damage means that any dose of radiation may cause some harm. Potential clinical effects of radiation exposure, in addition to those of malignancies, are listed on Table 5.1.
Table 5.1
Potential clinical effects of radiation exposure
Skin effects | Threshold dose (Gy) | Time of onset |
|---|---|---|
Early transient Erythema | 2 | 2–24 h |
Main erythema reaction | 6 | 1.5 weeks |
Temporary depilation | 3 | 3 weeks |
Permanent depilation | 7 | 3 weeks |
Dermal necrosis | >12 | >52 weeks |
Eye effects | ||
Lens opacity | >1–2 | >5 years |
Cataract | >5 | >5 years |
When considering the use of radiation, first one should ask if there is a non-radiation alternative that can provide the information desired, such as ultrasound. For many of the indications for obtaining a VCUG, the answer is no. Next, one should focus on decreasing the amount of radiation exposure to the patient. This is where the ALARA principle is applied.
Three factors affect the radiation dose to health care workers as well as to the patient for any radiographic procedure: time, distance, and shielding.
Time refers to the duration the patient is under exposure. Radiation exposure is directly proportional to the duration of fluoroscopic time so one should be mindful of the amount of time exposures are collected. Having protocols in place for the timing and number of images obtained during a VCUG can decrease the amount of radiation administered. For example, for a routine VCUG, there is little value in obtaining images over the ureters during filling or voiding unless an abnormality is seen. Time is decreased by having experienced personnel perform the procedure and teach those who are learning. The use of stopwatches, alarm bells, or radio communication letting the operator know how long the machine has been used for can decrease the exposure time [6, 7]. Pulsed fluoroscopy reduces radiation exposure by decreasing the amount of time the beam reaches the patient compared with continuous fluoroscopy.
Distance refers to separation of the subject from the radiation source. Increasing distance decreases the radiation dose in an inversely proportional fashion. The radiographic exam table should be as far from the radiation source as possible, although this is a fixed distance in fluoroscopy. The patient should be as close as possible to the image intensifier to reduce the scatter, although this may need to be modified due to patient fright and inability to cooperate. A less cooperative patient can lead to increased exam and exposure time due to motion artifact, causing the need to repeat images.
Shielding not only benefits the patient but also reduces the intensity of the radiation reaching the worker [7]. Shielding refers to limiting the direction of the radiation source in the housing and to collimating the beam size to minimize the x-ray exposure and scatter; it may include using lead pads to protect parts of the patient not involved in the examination and includes leaded rooms and aprons to protect workers in and outside of the fluoroscopy room.
As mentioned previously, the use of grids should only be used when the body part thickness indicates their use (when body part thickness is more than 12 cm) and should be removed when not indicated [8], which is common in pediatrics.
A combination of features can markedly reduce the radiation exposure. By using low-frequency pulsed fluoroscopy, tight collimation, no magnification, and half-dose parameters, the reduction in effective dose can be as high as 90 % without sacrificing resolution [3, 6, 9]. Using high-quality image-capture technology can also reduce radiation dose [9, 10]. Modern techniques have decreased the radiation dose of fluoroscopic VCUGs from 100 times to 10 times the radiation dose compared to radionucleotide cystograms [3], although the radiation dose may vary with fluoroscopic VCUGs pending the number of images obtained.
Digital Fluoroscopy
As technology continues to advance, digital fluoroscopy is becoming the mainstay. Digital radiology uses x-ray sensors bonded onto thin-film transistor integrated circuits to instantaneously convert the image stored on the sensor to a visible digital image [11]. This can be either through direct detection, converting the x-rays into an electronic charge, or indirect, by converting the x-rays into light and then converting the light into an electronic charge. In film, optical density on the film is used as an exposure indication. With digital images, overexposed images are not as easily identified as underexposed images, resulting in unknowingly increased radiation exposure [8]. Underexposed images are easily identified by increased noise. However, overexposed images are sharp, and exposure cannot be assessed visually. Up to 43 % of pediatric digital images are overexposed [11]. Feedback on the exposure is provided by the exposure indicator at the image receptor. It is influenced by the part thickness, artifacts, source-to-image distance, collimation, grids, centering, image plate size, and equipment and detector design [8]. There is a tendency towards exposure creep, the gradual increase in the technician’s selected exposure to obtain better quality images, as technicians need to repeat underexposed images [8]. In some areas, there is a trend towards exposure slide, where the radiologist accepts a nosier image until the image cannot be interpreted and needs to be repeated [5]. As a result, periodic checks by tracking exposure index and deviation index should be performed on a regular basis to assess for appropriate exposure.
Other challenges in digital imaging include the lack of standardization among vendors, lack of pediatric specific educational material and techniques, inability to use automatic exposure control in smaller children, and limitations of collimation and shielding of unnecessary body parts in smaller children [5]. Vendors of different machines have different exposure indicators; they have variable nomenclature and nonstandard units for reference values. In addition to the values being different, they may also be in inverse order so that the values may increase for overexposure for one manufactured and decrease for another [5]. Lastly, values provided may be inaccurate or uninterpretable in small children as indicated in Table 5.2. Technicians may be working with various types of machines in a given work day, making it more difficult to determine the amount of radiation being provided. These challenges are depicted on Table 5.3.
Table 5.2
Technical factors that can affect the exposure indicator (EI)
Size of detector/image receptor matched to patient size |
Collimation and field size in relation to patient’s body parts |
Metal implants in patients (scoliosis rods, metal plates, screws, pacemakers) |
Gonadal shield |
Table 5.3
Suggested guidelines for QC action based on estimated incident exposure (conventional CR system for an “equivalent” speed class = 200)
Fuji (S No.) | Agfa (IgM) | Kodak(exposureindex) | Detector exposure estimate (mR) | Indication |
|---|---|---|---|---|
>1,000 | <1.45 | <1,250 | <0.20 | Underexposed: repeat |
1,000–601 | 1.45–1.74 | 1,250–1,549 | 0.2–0.3 | Underexposed: QC exception |
600–301 | 1.75–2.04 | 1,550–1,849 | 0.3–0.7 | Underexposed: QC review |
300–150 | 2.05–2.35 | 1,850–2,150 | 0.7–1.3 | Acceptable range |
149–75 | 2.36–2.65 | 2,151–2,450 | 1.3–2.7 | Overexposed: QC review |
74–50 | 2.66–2.95 | 2,451–2,750 | 2.7–4.0 | Overexposed: QC exception |
<50 | >2.95 | >2,750 | >4.0 | Overexposed: repeat if necessary |
Despite these challenges, there are many advantages to digital radiography. The last image taken can be saved without having additional radiation exposure [3], post processing allows the radiologist to analyze various anatomical sites without needing additional images, the images can be stored and retrieved without losing image quality, and they can be accessed by various providers throughout a network.
Technical Considerations
Timing of VCUG
VCUGs needed for evaluation of a congenital anomaly can be obtained at any time. When a VCUG is necessitated following a urinary tract infection, previous recommendations were to delay the study by several weeks to allow the inflammatory process to resolve, as this may lead to detection of transient reflux secondary to ureteral dilation and inflammatory changes at the UVJ if performed too early [13, 14]. In addition, there also were concerns that if a VCUG is performed during an active infection, it may prolong the UTI or lead to bacterial dissemination and possibly sepsis [15]. Spencer [15] performed a retrospective review of VCUGs obtained over a 5-year period in all children admitted with a febrile UTI. Children were divided into two cohorts: those with an early VCUG, performed within the week of diagnosis after appropriate antibiotics had been initiated (67 patients), and those with a late VCUG, performed more than 1 week after diagnosis (65). All patients were followed for a period of 5 years after discharge to assess for complications. The incidence and severity of VUR were similar in the two groups. The early group showed no sign of worsening illness after the test was performed and had similar ED and hospital admissions rate as the late group. One hundred percent of the patients in the early group had their VCUGs done vs. 77 % in the late group (p = 0.005). These findings suggest that VCUGs can be obtained safely after appropriate antibiotics have been instituted and that compliance rates are better when the test is performed early, especially if it performed during an acute hospitalization. This could also be beneficial for children with recurrent episodes of pyelonephritis, who may only reflux during an active infection.
Patient Preparation
VCUGs are considered to be one of the most stressful urologic studies performed for both patients and parents [9, 16] with up to 27 % of patients experiencing severe distress [17] and 71 % of children experiencing serious or severe distress or panic on the Groningen Distress Rating Scale [16]. Distress may be secondary to the catheterization, but also may be related to fear of the unknown. Unexpected stress is more anxiety provoking and more difficult to deal with than anticipated or predicted stress [9]. Srivastava et al. [18] found that parents anticipated their child would experience more fear than they observed (p = 0.04), tended to anticipate that their child would suffer more distress than observed (p = 0.08), and anticipated that their child would suffer far more distress after the VCUG than they observed (p < 0.01). There was a significant correlation between the parents’ anxiety and their perception of the severity of their child’s fear (r = 0.52, p = 0.009), distress (r = 0.48, p = 0.017), and pain (r = 0.50, p = 0.01) during the procedure.
Preparation of both the parent and the child via education can help decrease perceived anxiety and stress levels for the parents and the child [19, 20]. Education can be in the form of reading materials or videos. In some centers, children are given a tour and allowed to sit on the table while a doll is catheterized as the procedure is explained so they know what to expect and are given an opportunity to ask questions. After determining that VCUGs were one of the most stressful studies performed at their institution partly due to lack of information, Phillips et al. [16] sought to determine if better patient preparation would alleviate parental and patient distress. They had two intervention groups and compared their distress level to their historic controls. One intervention group was given a story book describing the procedure and expectations, while the second group was given the book as well as play therapy where they had the procedure explained with the aid of a gender appropriate doll. The child’s distress was significantly reduced with preparation (p = 0.0026) with a median distress score on the Groningen Distress Rating Scale of 2.5 in the preparation group vs. 3.3 in the controls (Fig. 5.2). Among the preparation group, children had lower distress scores when parents provided full disclosure compared to those where parents avoided or omitted upsetting details (p = 0.0025) (Fig. 5.3). Between the two preparation groups, there was no difference in distress scores with the addition of play therapy.
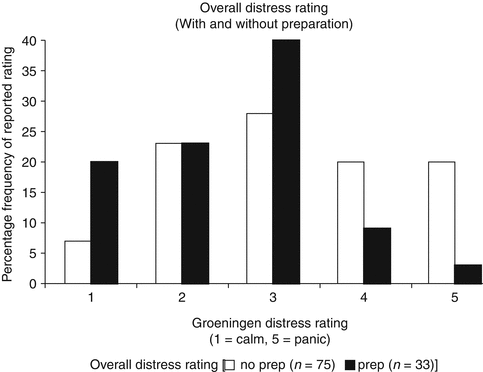

Fig. 5.2
During the procedure, a calm parent should be allowed to accompany the child as this decreases patient distress [16]. Parents can help their child by providing support and distracting them with toys, videos, balloons, etc. In addition, children depend on their parents to help them cope with stressful situations [16]. Children have been found to be more cooperative and calm with invasive procedures when supported by their parents and prompted to model coping behaviors [16]. The use of child life specialists when available is also recommended.
The Role of Sedation
Despite appropriate preparation, some patients may still exhibit high levels of distress when having a VCUG, particularly if they have had a previous negative experience such as prior catheterization or if there are other external circumstances. During these situations, sedation may be useful. There are several concerns regarding the use of sedation: cost, time, safety, and effects sedation can have on bladder dynamics. Herd found that the increased costs were related to the need for a recovery room and staff and that safety was excellent in the context of experienced staff with proper equipment and available emergency preparations [21]. In a randomized trial of sedation vs. placebo, sedation added approximately 60 min to the entire procedure, mostly as recovery time [22]. There was no significant difference in the filling or voiding phase, and there was no difference in the volume infused between the two groups [22]. VUR was identified in 16 % of children, without difference between the two groups (p = 0.31).
Although the use of sedation increases the overall procedure time, sedation is useful in decreasing anxiety and distress among children older than 1 year and their parents. In a randomized double-blind controlled trial of oral midazolam 30 min prior to catheterization at a dose of 0.5 mg/kg vs. placebo, 61 % of children experienced serious or severe distress in the placebo vs. 16 % in the treatment group, with the NNT being 2.9 (Fig. 5.4) [22]. When the test was divided into parts (entering the room, catheterization, filling, voiding, and leaving the room), there were differences in distress detected at entering the room (p = 0.01), filling (p = 0.0001), and voiding (p < 0.0001), and no difference detected at catheterization (p = 0.10) and leaving the room (p = 0.18) as depicted in Fig. 5.5. Both groups were provided educational pamphlets and offered play therapy prior to the procedure. In review of five comparative studies, the use of midazolam significantly lessened distress without affecting voiding dynamics or having adverse effects [21]. Ferguson randomized children to midazolam 15 min prior to the procedure vs. placebo in a double-blind fashion and found no difference with the use of midazolam at decreasing anxiety in children or in their secondary outcomes of the degree of parental anxiety, health care professional perception, and post-procedure behavior outcomes [23]. It is possible that the differences may be secondary to a smaller sample size, decreased wait time to the procedure, or the use of different assessment tools.
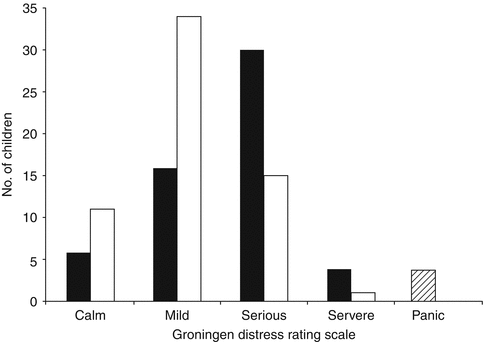
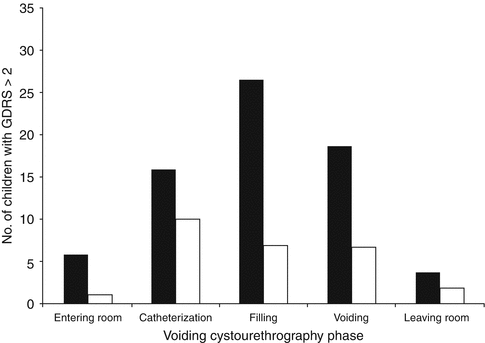

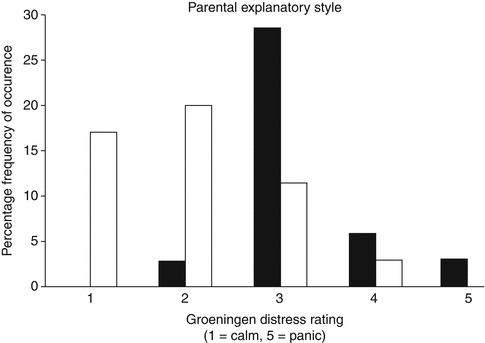
Fig. 5.4
Distress levels at any stage during voiding cystourethrography [22]. Bar graph shows peak distress score at any stage during voiding cystourethrography (VCU) (n = 117). Fifty-six received placebo (black bars) and 61 received midazolam (white bars). Striped box represents four children – all of whom were in the placebo group – who failed to complete VCU due to panic (From Herd et al. [22]. Reprinted with permission from the American Journal of Roentgenology)

Fig. 5.5




Distress levels at different phases of a voiding cystourethrography [22] Bar graph shows number of children (n = 117) who experienced serious or severe distr ess (Groningen Distress Rating Scale [GDRS] score > 2) at each phase of voiding cystourethrography. Fifty-six received placebo (black bars) and 61 received midazolam (white bars) (From Herd et al. [22]. Reprinted with permission from the American Journal of Roentgenology)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree