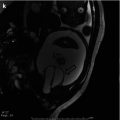
Fig. 12.1
Acute pyelonephritis in the upper pole of the left kidney of a 5-week-old male with urosepsis. (a) Longitudinal sonogram shows hyperechoic left upper pole with loss of corticomedullary differentiation (arrow). (b) DMSA scan obtained the same day demonstrates decreased uptake in the left upper pole without loss of volume (arrow)
Several clinical studies have clearly demonstrated that renal sonography is not as reliable as DMSA scintigraphy for the detection of APN [26–32]. In one prospective study of 91 children with culture-documented febrile UTIs, DMSA renal scans showed changes consistent with APN in 63 % of patients, while sonography revealed changes consistent with APN in only 39 % of the same patients [26]. In another prospective study of 112 children with their first documented symptomatic UTI, ultrasound was effective in detecting dilatation of the collecting system and renal swelling but failed to detect over half of those patients with DMSA evidence of APN [27] (Fig. 12.2).
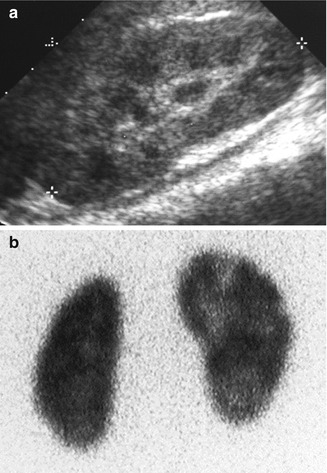

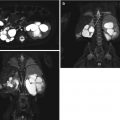
Fig. 12.2
False-negative sonogram of the right kidney of a child with multifocal acute pyelonephritis. (a) Longitudinal sonogram of the right kidney shows normal echogenicity and corticomedullary differentiation. (b) DMSA scan demonstrate swollen right kidney with multiple foci of decreased uptake
Power Doppler Sonography
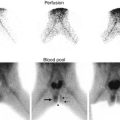
As focal ischemia is an early event in APN, it seems logical to expect high sensitivity for Doppler sonography in its detection. The classic color Doppler ultrasound of the kidney outlines the speed and direction of moving blood through the larger intrarenal blood vessels but does not visualize smaller cortical vessels resulting in poor sensitivity in detection of APN. In contradistinction, power Doppler sonography displays the strength of the Doppler signal from all moving blood cells regardless of speed or direction and is therefore more sensitive than color Doppler for detection of blood flow in the small vessels (Fig. 12.3). Stogianni et al. described effective use of power Doppler in the diagnosis of APN utilizing linear 5–10 MHz transducers in children less than 3 months old and convex 5 MHz transducers in children older than 3 months [33]. Axial and longitudinal images were obtained to develop intricate vascular maps of the kidneys. APN was defined by the decreased or absent blood flow in specific zones of the renal parenchyma as well as renal swelling and loss of corticomedullary differentiation [33] (Fig. 12.4).
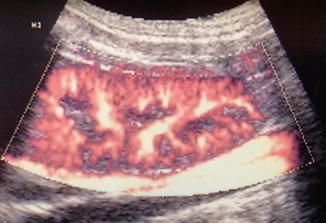
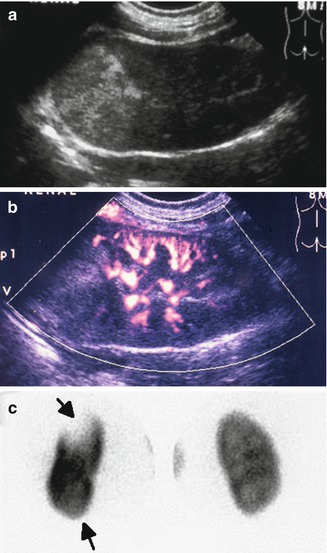

Fig. 12.3
Normal power Doppler renal sonogram. Coronal image of the right kidney of a piglet shows normal perfusion of the renal parenchyma

Fig. 12.4
Abnormal power Doppler renal sonogram and DMSA scan of a child with left acute pyelonephritis. (a) Grayscale longitudinal sonogram of the left kidney shows hyperechoic upper pole, but no definite abnormality in the lower pole. (b) Power Doppler sonogram demonstrates decreased/absent perfusion of the upper and lower poles. (c) DMSA scan shows a large photopenic area in the upper pole of the left kidney and mild decreased uptake in the lower pole (arrows)
Recently Brader et al. retrospectively studied a combination of grayscale and power Doppler renal sonography in children with clinical APN and compared the findings with DMSA scans [34]. Expanding criteria for APN to include changes in flow characteristics on power Doppler in addition to changes on grayscale sonography, they found combined sensitivity of 92.1 %, specificity of 97.8 %, and positive predictive value of 88–92 %. When compared with DMSA scan, they found sensitivity and specificity of 94 and 100 %, respectively. However, in an experimentally induced APN in piglets, sensitivity of power Doppler for the detection of histopathologically confirmed lesions was significantly lower than DMSA SPECT, CT, and MRI [35].
Renal Cortical Scintigraphy
Renal cortical scintigraphy with 99m Tc dimercaptosuccinic acid (DMSA) is considered the imaging modality of choice for detecting renal parenchymal involvement in children with UTI and as a marker to assess the extent and progression of renal damage [36]. Approximately 60 % of administered radiolabeled DMSA is picked up by the proximal tubular cells, and the remaining is filtered and excreted at a low concentration. Therefore, the delayed images show excellent visualization of the renal cortex without any significant tracer activity in the pelvicalyceal systems.
Approximately 2 h after intravenous administration DMSA (≤0.05 mCi/kg body weight; minimum 0.03 mCi, maximum 3 mCi), images of the kidneys are obtained. Basically two imaging techniques can be used: planar imaging with magnification or single-photon emission computerized tomography (SPECT).
For planar imaging with pinhole magnification, a high-resolution, parallel-hole collimator is used to obtain posterior image of both kidneys together for calculation of differential renal function. In addition posterior and posterior-oblique images of each kidney are acquired using a pinhole collimator with 3–4 mm aperture insert (Fig. 12.5).
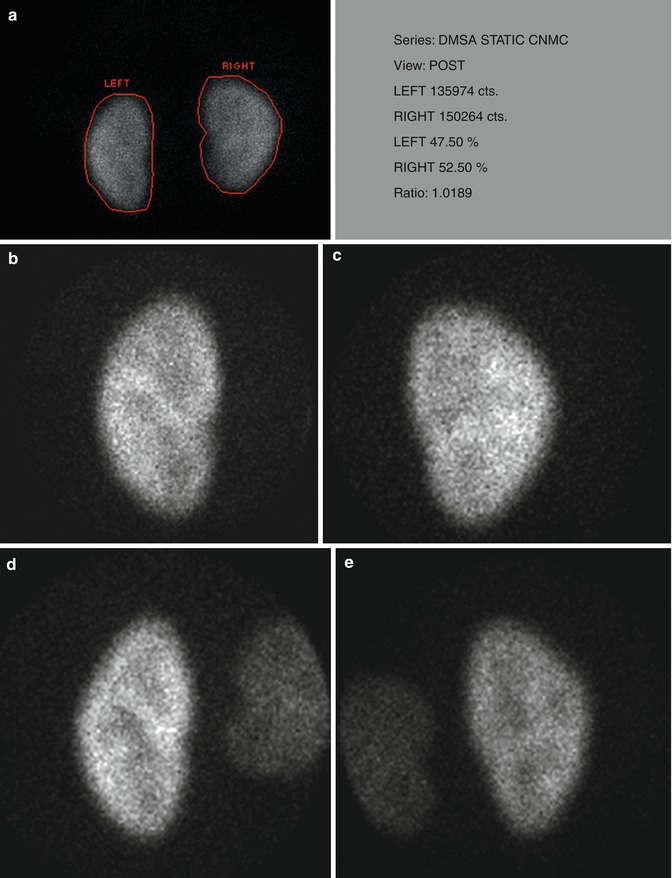

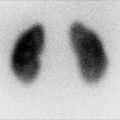
Fig. 12.5
Normal planar DMSA scan with pinhole magnification in a 5-year-old child. (a) Posterior image using parallel-hole collimator shows normal cortical uptake bilaterally with the calculated differential function of 47.5 % on the left and 52.5 % on the right. (b, c, d, and e) Magnified pinhole posterior and posterior-oblique images of the kidneys show normal cortical uptake of DMSA with relative central photopenia corresponding to the medulla and collecting systems
For SPECT imaging, usually a dual detector rotating gamma camera equipped with high-resolution, low-energy collimators is used to acquire 120 images of the kidneys (3° apart). Data acquisition takes approximately 20 min. The images are then reconstructed in coronal, transverse, and axial planes (Fig. 12.6).
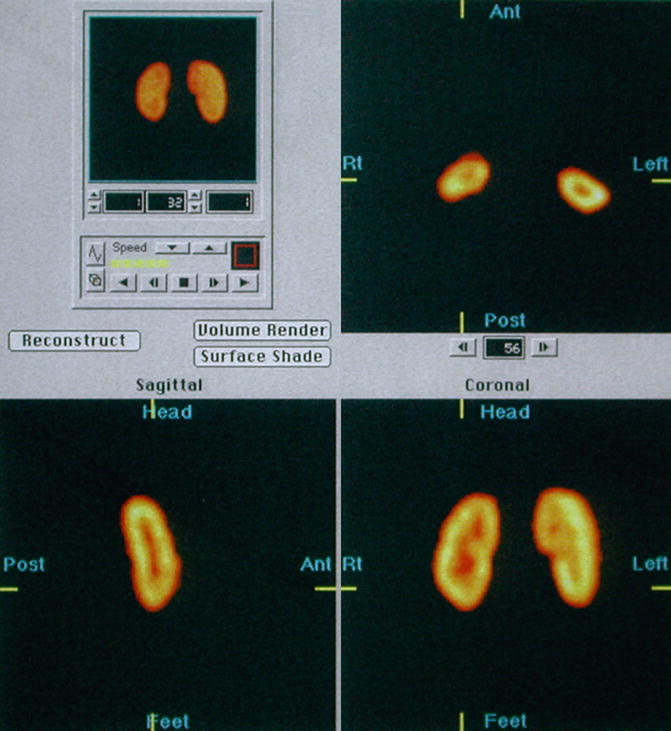

Fig. 12.6
Normal SPECT: selected reconstructed transverse, sagittal, and coronal images show normal cortical DMSA uptake
Normal DMSA Scan
Uptake of DMSA in normal kidneys reflects the morphology of renal cortex. High-resolution images show the details of the cortex and cortical columns with good differentiation from the collecting systems and medulla. Irregularities in the contour of the kidneys due to fetal lobulation may be present between the medullary pyramids (over cortical columns) and can be differentiated from the cortical scars which occur over the pyramids (between cortical columns) (Fig. 12.7).
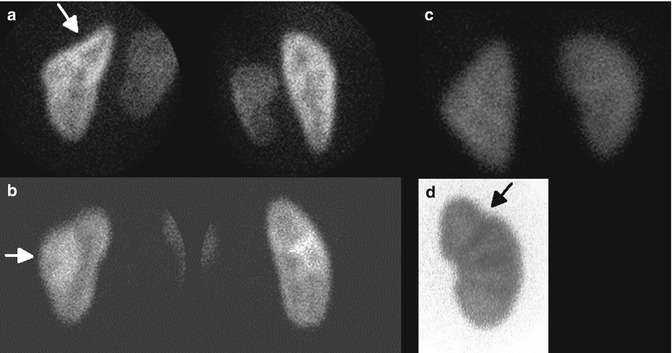

Fig. 12.7
Normal variants on DMSA scan: splenic impression (arrow) (a), column of Bertin (arrow) (b), triangular left kidney (c), and fetal lobulation (arrow) (d). Note cortical column under the fetal lobulation
Acute Pyelonephritis and Renal Scar
The typical manifestation of APN on DMSA scan is decreased cortical uptake, usually focal or multifocal, without volume loss or contraction of the renal cortex. In severe cases diffusely decreased uptake of DMSA may be observed (Fig. 12.8).
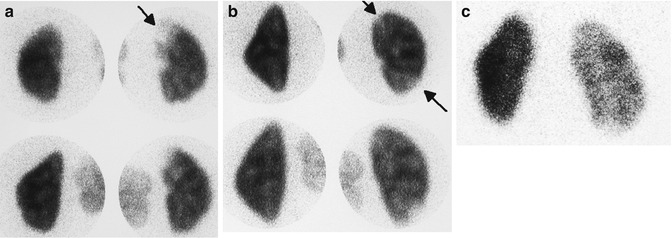

Fig. 12.8
Scintigraphic patterns of acute pyelonephritis: acute pyelonephritis usually presents either as a single focus (a) or multiple foci (b) of decreased uptake without volume loss (arrows). A less common pattern is panpyelonephritis manifesting as diffusely decreased uptake in an enlarged kidney (c)
A mature cortical scar is usually associated with contraction and loss of volume of the involved cortex manifested as wedge-shaped defect, cortical thinning, or flattening of the renal contour (Fig. 12.9).
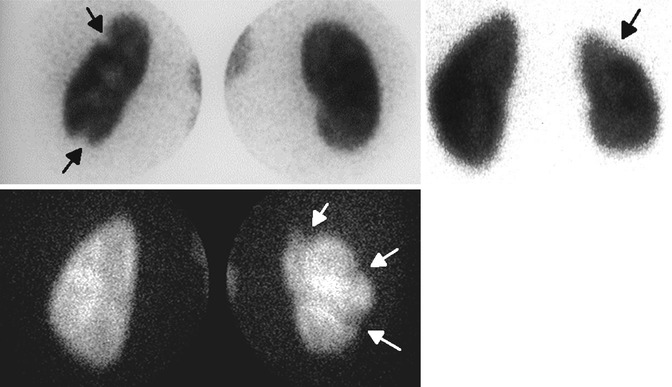

Fig. 12.9
Scintigraphic patterns of cortical scars in three different patients: cortical scars appear either as focal areas of decreased uptake with volume loss, cortical thinning, or as a more extensive polar or global volume loss (arrows)
Sensitivity and Specificity of DMSA for Detection of Acute Pyelonephritis
Early clinical reports showed that renal cortical scintigraphy using 99m Tc DMSA was significantly more sensitive than intravenous urogram (IVU) and renal sonography in the detection of APN [37–39]. To evaluate the true sensitivity and specificity of renal cortical scintigraphy for the detection and localization of APN, studies were conducted in a piglet model of surgically inducing unilateral vesicoureteral reflux of infected urine and using strict histopathologic criteria as the standard of reference [40, 41]. Planar DMSA scans using pinhole magnification were highly reliable for detecting and localizing experimental APN with a sensitivity of 87–89 % and specificity of 100 % in both studies. When individual pyelonephritic lesions were analyzed, DMSA scan findings correlated with histopathological changes with an agreement rate of 89–94 %. Those lesions not detected were microscopic foci of inflammation not evident on gross examination and not associated with significant parenchymal damage.
Not only is DMSA scintigraphy highly sensitive and specific for the diagnosis of APN, but it also provides important information regarding renal function and the extent of renal parenchymal inflammation. Documentation of renal parenchymal damage associated with APN is fundamental to understanding the relative roles of infection and vesicoureteral reflux in the etiology of pyelonephritis and renal scarring.
Pinhole Versus SPECT Imaging
In some clinical studies, SPECT imaging is reported to be more sensitive than pinhole imaging for detecting APN [42–44]. However, in experimentally induced pyelonephritis in piglets, using histopathologic criteria as the standard of reference, the sensitivity and specificity for detecting affected renal zones were 86 % and 95 % for pinhole imaging and 91 % and 82 % for SPECT. The overall accuracy was 88 % for both techniques in assessing kidney involvement [45] (Fig. 12.10). Thus, SPECT imaging appears to be slightly more sensitive than standard pinhole imaging but may result in more false-positive findings. Furthermore, it may be easier to differentiate acute inflammatory changes from chronic renal scarring with pinhole imaging.
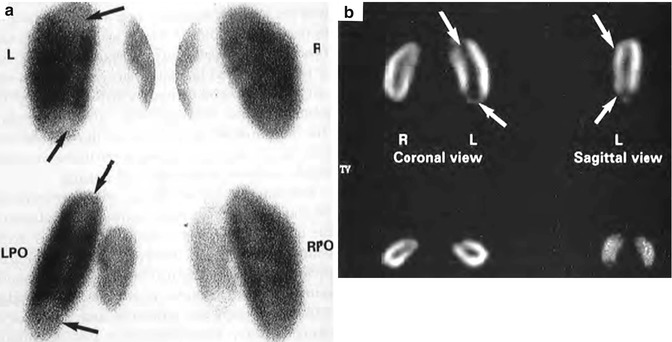

Fig. 12.10
(a) Posterior-oblique pinhole and (b) coronal and sagittal SPECT images of 99mTc-DMSA renal scan in a young pig with bilateral vesicoureteral reflux and acute pyelonephritis 48 h after introduction of E. coli broth into the bladder. Photopenic lesions in the left upper and lower poles are demonstrated by both techniques (arrows) (From Pohl et al. [142]. Reprinted with permission from Informa Healthcare)
MAG 3 Renal Scan
Technetium-99 m labeled mercaptoacetyl triglycine is a renal imaging radiopharmaceutical which is rapidly picked up and secreted by the tubular cells yielding excellent visualization of pelvicalyceal systems and ureter making it the radiopharmaceutical of choice for dynamic renal imaging such as diuresis renography and captopril renography. However, its high cortical concentration during the first few minutes after injection and subsequent rapid clearance may allow detection of focal cortical functional abnormalities. The expected finding in APN would be focal decreased uptake on the early images with subsequent poor clearance (cortical retention) in the same area.
In a clinical comparative study, Piepz et al. concluded that the accuracy of the MAG3 renal scan was population dependent. When the DMSA scan was normal or very abnormal, the MAG3 image correctly reflected the findings of the DMSA renal scan. However, when the DMSA abnormalities were less pronounced, the early MAG3 scan failed to detect about half of the cases [46].
Computed Tomography (CT) Scans
Abdominal CT scanning is an effective rapid imaging technique for documenting the nature and extent of renal parenchymal involvement and for evaluation of perinephric space as well as other abdominal viscera. However, because of high radiation dose and the need for rapid IV administration of iodinated contrast media, its routine use in evaluating children with UTI is impractical and should be reserved for complicated cases.
APN lesions typically appear as wedge-shaped, ill-defined, or striated areas of decreased attenuation (Fig. 12.11). In an experimental refluxing piglet study performed by Majd et al., the CT scan proved to be highly accurate for the detection of pyelonephritic lesions with sensitivity and specificity similar to DMSA SPECT and MRI [35].
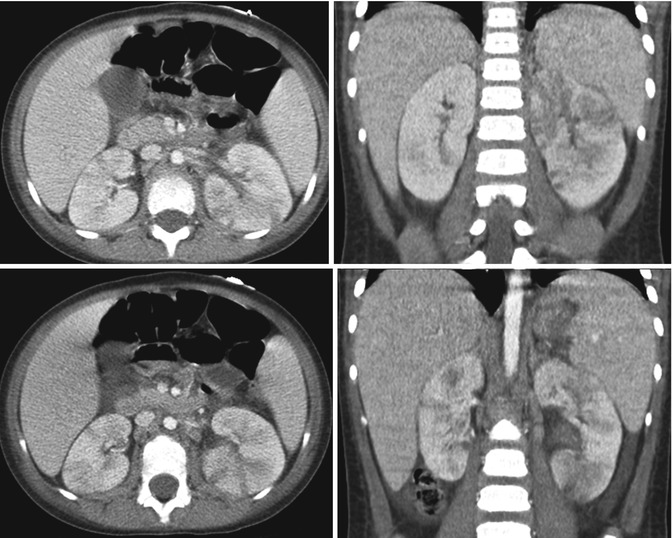

Fig. 12.11
Bilateral multifocal acute pyelonephritis in a 4-year-old female with fever, abdominal pain, and shock. Transverse and coronal CT images demonstrate multiple striated areas of hypoattenuation in the swollen left kidney and less severe involvement of the right kidney
Magnetic Resonance Imaging (MRI)
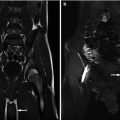
Magnetic resonance imaging (MRI) with IV administration of gadolinium has been shown to be highly accurate for the detection of pyelonephritic lesions both in clinical and experimental studies. The fast inversion recovery sequence markedly decreases the signal intensity of normal parenchyma and allows pyelonephritic lesions to be seen as foci of medium or high signal intensity (Fig. 12.12). In a clinical study, the sensitivity of MRI was similar to that of DMSA scan [47]. In an experimental refluxing piglet study, MRI showed high sensitivity and specificity for detection of pyelonephritic lesion similar to DMSA SPECT and CT [35] (Table 12.1).
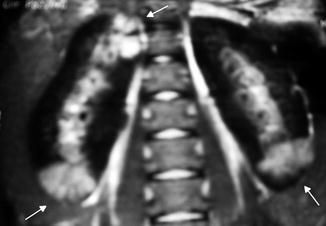

Fig. 12.12
MRI manifestation of acute pyelonephritis: coronal post-gadolinium fast multiplanar inversion recovery images demonstrate foci of high signal intensity in the upper and lower poles of the right kidney and the lower pole of the left kidney against a background of low signal in the normal cortex (arrows)
Table 12.1
Diagnosis of experimental acute pyelonephritis in piglets: comparison of DMSA, MRI, CT, and power Doppler
SPECT | Gadolinium | Spiral | Doppler | |
|---|---|---|---|---|
DMSA (%) | MRI (%) | CT (%) | Sono (%) | |
Sensitivity | 94 | 91 | 87 | 56 |
Specificity | 98 | 92 | 92 | 81 |
Overall accuracy | 96 | 91 | 90 | 69 |
Renal scars appear as a focal area of parenchymal volume loss with deformity of the renal contour and can be easily differentiated from APN [48]. Renal function can be estimated on MRI without ionizing radiation and iodinated contrast media. In one study of children with febrile UTIs, the cortical phase of contrast-enhanced MRI demonstrated greater interobserver agreement and cost-effectiveness when compared with DMSA for the diagnosis of pyelonephritis [10].
Fungal Infections
Renal fungal infections are most often recognized in high-risk groups of neonates and infants. Reported mortality rates in children with candidemia range from 19 to 31 % and with invasive aspergillosis from 68 to 77 % [49]. Premature, low-birth-weight infants are at highest risk for disseminated candidiasis secondary to immature immune systems, increased invasive procedures, use of H2 blockers, and multiple courses of antibiotics [50].
Renal candidiasis is a combination of candiduria and ultrasound evidence of renal parenchymal infiltration or fungal bezoars in the collecting system. Fungal bezoars are a rare complication that may cause urinary obstruction [51]. In neonates with candiduria, the reported incidence of renal candidiasis varies between 35 and 58 %, whereas patients with candidemia have a 61–70 % prevalence of candiduria and 5–33 % incidence of renal candidiasis [51]. Term infants and older children with congenital abnormalities of the urinary tract are also at higher risk for candiduria [52].
The presentation of candiduria may be asymptomatic or as an acute urinary infection with symptoms of dysuria, cloudy urine, fever, or failure to thrive. Currently there is no standard definition used for diagnosis of candida UTI; however, most of the reported literature uses 104 colony-forming units/mL via catheterized specimen. Obstructive renal candidiasis may present with sepsis, acute renal failure, or palpable flank mass. The clinical presentation of systemic candidemia is similar to bacterial sepsis with lethargy, feeding intolerance, apnea, and/or respiratory distress [51]. Neonatal candidemia often involves multiple organ systems. Due to the invasive nature of this disease work-up should be performed to rule out systemic fungal infection if candida UTI is found. This includes blood, urine, and cerebral spinal fluid cultures, eye examination, echocardiogram, and imaging of the liver, spleen, and kidneys [53].
Imaging of Renal Fungal Infections
Sonography and IVP
Renal ultrasonography is performed routinely in cases of candiduria and systemic candidemia. In obstructive renal candidiasis dilation of the upper urinary tract is visualized as well as fungal masses (bezoars). It is possible to see focal or diffuse parenchymal changes in nonobstructing renal candidiasis [51]. Fungal masses on ultrasound are hyperechoic structures within the collecting system that do not have acoustic shadowing [54] (Fig. 12.13). The differential for these findings includes urolithiasis, nephrocalcinosis, clot, debris, and tumor. Fungal infiltration of the kidney may be seen on ultrasound in the presence of positive urine and/or blood cultures. The ultrasound findings are characterized by enlarged kidneys with diffusely increased echogenicity [55].
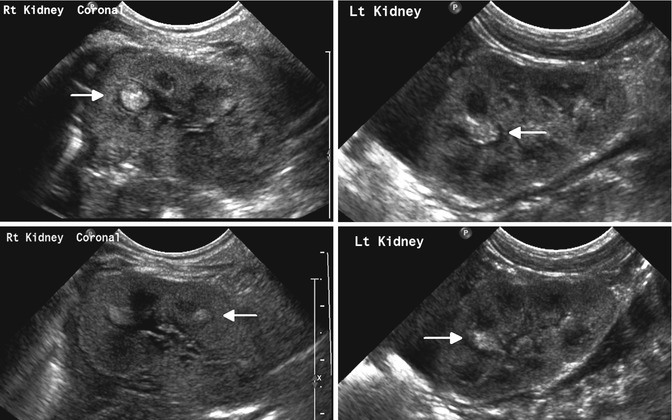

Fig. 12.13
Bilateral renal fungal infection in an infant with candidemia: sonogram shows multiple discrete nonshadowing echogenic foci within the calyces of both kidneys (fungus balls)
DMSA
Use of DMSA for renal candidiasis was reported in one case study, and it did not aid in diagnosis but did demonstrate decrease uptake and corresponding renal parenchymal damage [56]. In cases where fungal bezoars persist following treatment and lead to reinfection, DMSA may be helpful in determining differential renal function. If the affected kidney is poorly functioning, then surgical planning for nephrectomy, endoscopic manipulation, or percutaneous resection may be considered.
CT Scans
Although ultrasonography is used more commonly in critically ill neonates, occasionally CT scans have been used to diagnose renal candidiasis. The appearance of fungal bezoars is that of a soft tissue masses in the collecting system [55]. CT scan with delayed urogram was reported to assist in the rare diagnosis of a neonate with candida infection and urinary ascites secondary to fungal obstruction and forniceal rupture [57].
MRI
MRI imaging was reported to be superior to CT scan for imaging of fungal balls in the collecting system. On the T2-weighted phase and STIR (short TI inversion recovery) images, the bezoars were hyperintense compared to renal parenchyma [58]. While this modality is excellent for demonstrating fungal bezoars, it often requires anesthesia and is a lengthy test that is risky in critically ill patients such as septic neonates. Other studies have shown, however, that MRI imaging was less effective than ultrasound at detecting fungal bezoars [56].
Tuberculosis (TB)
Genitourinary tuberculosis (GUTB) is the second most common site of extrapulmonary tuberculosis. While 90 % of GUTB cases occur in non-westernized populations where TB is still endemic [59], a resurgence is anticipated in western countries due to the spread of HIV and acquired immunodeficiency syndrome (AIDS) [60]. The classic presentation of renal TB is kidney damage from obstruction or massive caseous destruction [62]. TB infection occurs through inhalation of aerosolized Mycobacterium tuberculosis bacilli. The kidneys are infected through hematogenous spread of the bacilli. The bacilli pass down the renal tubules involving the renal calyces and can eventually enter the renal pelvis and attach to the urothelium. Stricture formation commonly results in hydroureter or hydronephrosis, and up to 10 % of patients with renal TB have bladder contractures [59]. In addition, GUTB may spread to the prostate and epididymis.
Imaging of Renal Tuberculosis
Sonography and IVP
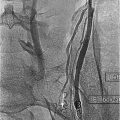
Ultrasonography is the least specific imaging technique when compared with IVU and CT scan, particularly when evaluating the renal parenchyma. It is still particularly useful in diagnosis of epididymal involvement and TB orchitis [67]. IVP is similar to CT scan in the ability to demonstrate focal scars and hydronephrosis and is reported to be superior to CT scan for showing abnormalities of the bladder [59]. In late-stage disease, IVP demonstrates distortion of the calyces, strictures of the ureters, and bladder fibrosis [63]. Irregularities of the calyceal or parenchymal contour, calcification, or autonephrectomy can also be demonstrated [59] (Fig. 12.14).
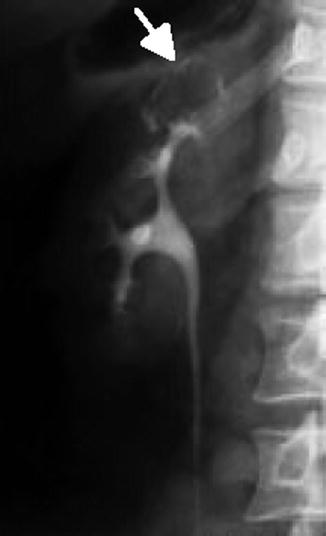

Fig. 12.14
IVP demonstration of renal tuberculosis: a calcification rimmed cavity does not fill with IV contrast (arrow) (From Patterson et al. [143]. Reprinted with permission from Blackwell Publishing)
CT Scans
CT scan has the benefit of demonstrating abnormalities and lesions within and outside the urinary tract that are suggestive of GUTB. CT scan is the most sensitive method for detecting renal calcification. It is also best for detection of thickening, fibrosis, and ulceration along the renal tract. Findings of thickening and fibrosis of the bladder wall may be suggestive of tuberculosis cystitis [59]. Due to the rarity of pediatric renal TB, there is a paucity of literature regarding routine diagnostic use of CT scan in children for TB (Fig. 12.15).
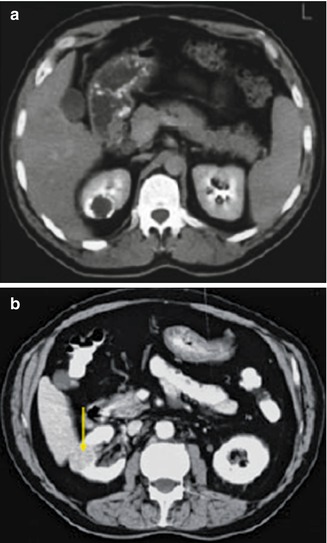

Fig. 12.15
CT scan in same patient as Fig. 12.14: (a) CT scan with IV contrast shows a calcified caseation of an upper pole lesion which does not fill with contrast. (b) CT nephrogram phase showing a calcified granulomatous lesion (arrow) (From Patterson et al. [143]. Reprinted with permission from Blackwell Publishing)
DMSA
A DMSA scan may be helpful in guiding surgical management of patients who are being evaluated for nephrectomy. If a nonfunctioning kidney is present often nephrectomy will be performed to eradicate disease.
MRI
Due to the cost limitations and availability of MRI in areas where GUTB is prevalent, MRI has been a rarely used imaging modality.
Renal Abscess
Renal abscess is a rare form of pediatric infection. The three basic pathophysiologic mechanisms of renal abscess formation are hematogenous spread, ascending infection, and contamination by proximity to an infected area [68]. In the past, the majority of renal abscesses were not thought to be caused by ascending infection. Historically, Staphylococcus aureus was the most common reported offending agent presumably as a result of hematogenous seeding from a peripheral cutaneous site of origin [69, 70]. More cases of gram-negative infections in the presence of vesicoureteral reflux or other anatomic abnormalities of the urinary tract are now being seen [71].
Abscesses can originate in the corticomedullary portion of the kidney, the renal cortex, or within layers of Gerota’s fascia [68]. Symptoms of renal abscess are often nonspecific which may lead to a delay in treatment. It can mimic symptoms of appendicitis or, when a palpable mass is present, may be mistaken for neoplasm, i.e., Wilms’ tumor or neuroblastoma. The most common presentation of a renal abscess includes high fever, lethargy, and flank pain associated with laboratory findings of leukocytosis and elevated ESR. Dysuria and/or foul-smelling urine is not regularly seen with initial presentation, and the patient may have sterile urine cultures, particularly in cases of hematogenous seeding. A variety of imaging techniques have been used to diagnose renal abscesses, including IVP, angiography, gallium-67 scintigraphy, sonography, and CT [72–76].
Imaging or Renal Abscesses
Sonography and IVP
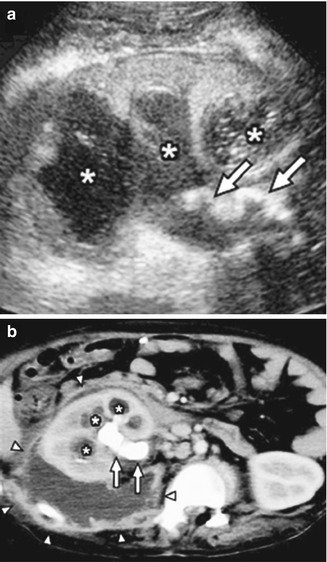
Traditionally IVP and occasionally angiography were used to diagnose a renal abscess [69]. Findings suggestive of renal abscess were intrinsic masses with calyceal deformity and diminished renal function. However, with advances in imaging, ultrasound and CT have become the most common diagnostic imaging for renal abscesses [76]. In children, ultrasound is the primary imaging modality of choice to help limit radiation exposure. Typical findings on renal ultrasound consistent with renal abscess are presence of anechoic or septated renal masses [76] (Fig. 12.16). Ultrasound may also be used to assist therapeutically; ultrasound-guided sampling for culture of a renal abscess can help dictate specific antibiotic therapy. It can also be used to facilitate treatment with complete abscess percutaneous aspiration and/or drain placement. Ultrasound is also useful for follow-up and determining resolution of abscess during the course of treatment as it is noninvasive and does not expose the patient to radiation.
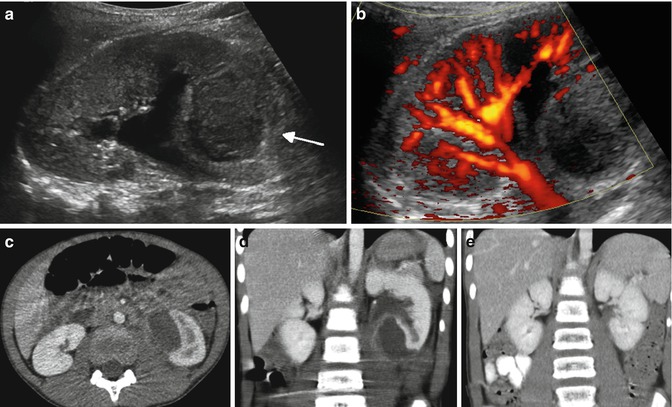

Fig. 12.16
Subcapsular abscess in a 6-year-old male with flank pain and low-grade fever: the renal sonogram shows a circumscribed area of mixed echogenicity (arrow) (a) with absent perfusion on Doppler image (b). Transverse (c) and coronal (d) CT images demonstrate a large collection of fluid compressing the medial aspect of the lower pole of the left kidney. Follow-up CT scan (e) 2 weeks after percutaneous drainage and intravenous antibiotics shows complete resolution of the abscess and re-expansion of the renal parenchyma
VCUG
VCUGs are often obtained in children with renal abscesses as part of an evaluation to determine if any genitourinary abnormality is present. This information is necessary for prevention and management of future infections. A VCUG is not useful in acute diagnosis of renal abscess and is contraindicated at time of infection. When VUR is associated with renal abscess, the causative organism is often found to be gram-negative bacteria [69].
CT Scan
Although CT scans are less often used in children, renal abscess cavities can have varying levels of necrosis and septation, at times, making it difficult to distinguish from renal neoplasm on sonography. CT scans maybe utilized for clarification when MRI is not available or sedation of the patient is not desired [77]. Findings on CT scan consistent with renal abscess are well-defined areas of low attenuation which develop a rim of peripheral enhancement after infusion of intravenous contrast; however, the lesion itself does not enhance [76] (Fig. 12.16).
MRI
At this point in time, there are no studies in children demonstrating a definitive benefit of MRI for managing renal abscesses and is therefore not being used routinely in their evaluation. The use of MRI has been reported in the adults where lesions are concerning for malignancy and need to be distinguished from abscess.
Treatment of Renal Abscess
The classic treatment of renal abscess has been surgical drainage in addition to appropriate antibiotic therapy. However, improved antibiotics and diagnostic techniques, together with the ability to obtain culture by percutaneous aspiration or drainage under ultrasonic control, have often obviated the necessity for surgical intervention. Currently, most cases of renal abscess initially can be managed initially with parenteral antibiotics, with percutaneous drainage being reserved for persistent infection. If percutaneous drainage of the renal abscess and antibiotic treatment fail to successfully eradicate the abscess, open exploration or even nephrectomy may be necessary.
Xanthogranulomatous Pyelonephritis
Xanthogranulomatous pyelonephritis (XGP) was first reported in 1916 by Schlagenhaufer [78]. The etiology of XGP remains unknown but is often associated with urinary tract obstruction, infection, and/or renal stones. XGP is an atypical form of severe chronic renal parenchymal infection characterized by unilateral destruction of parenchyma and accumulation of lipid-laden macrophages either surrounding abscess cavities or as discrete yellow nodules. While it predominately affects middle-aged women, it may occur in all ages. It is rare in children with only 265 reported cases since 1960 [79]. The age of presentation has ranged from infancy to 16 years with the most common age of presentation less than 8 years old and rare presentations in infancy [78]. XGP is focal in only about 10 % of cases and is most commonly caused by Proteus mirabilis or Escherichia coli infection with 50–75 % of patients having positive urine cultures [78, 80].
Most patients present with nonspecific symptoms of chronic infection, including weight loss, recurrent fever, failure to thrive, pallor, and lethargy, although those with the focal form often appear healthy [81, 82]. Urinary symptoms are uncommon, and less than half of the patients present with hypertension [83]. A palpable abdominal mass is present in approximately one-third of cases.
Both diffuse and focal forms of the disease have been reported, with the focal form being more common in children [81, 84]. Calcification or stones may be present in 70–79 % of patients with staghorn calculi being common, although this is less often seen in the focal XGP [78]. In children less than 8 years of age, the disease is usually focal, unilateral with left predominance, and without calculus [78]. The pathologic and radiologic differences between focal and diffuse XGP have been described [86]. No radiologic feature is diagnostic of XGP.
Imaging of Xanthogranulomatous Pyelonephritis
Sonography and Intravenous Pyelography (IVP)
In the diffuse form of XGP, the typical ultrasound findings are increased renal size, replacement of normal parenchyma with multiple fluid-filled masses, and posterior acoustic shadowing due to calcifications [87]. In the focal form, hypoechoic areas due to distended calyces and an inflammatory mass with a central hyperechoic area consisting of granulomatous tissue are noted [78].
Power Doppler
It has been suggested that use of a power color Doppler may be useful in the differentiation of XGP from neoplasm. In a retrospective examination of patients found to have XGP following nephrectomy, it was noted that both patients had lack of perfusion for the expanding intrarenal process [88]. This is directly contrasted to a renal neoplasm in which increased perfusion is found on color Doppler.
DMSA or MAG-3 Scintigraphy
CT Scan
Characteristic sonographic or CT appearances of XGP also have been reported [89]. Typically, this is characterized by dilated calyceal spaces producing the “bear paw sign” [78]. CT scan enables excellent cross-sectional imaging which helps determine extrarenal extension. Some studies have demonstrated that four-phase CT scan is as accurate as MRI for the preoperative diagnosis of XGP [90] (Fig. 12.17).