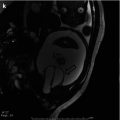
Fig. 18.1
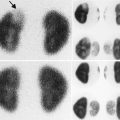
A 2 ½-year-old with a history of “megaureter” being followed for upper tract dilation. There is significant progression of left-sided dilation between September 30, 2008 (top), and February 23, 2009 (bottom)
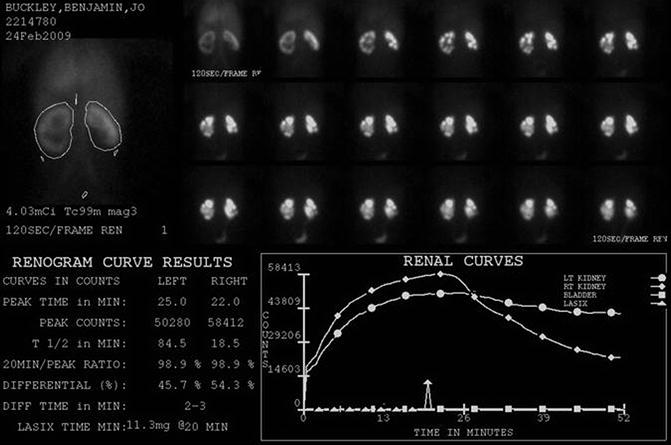
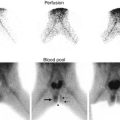
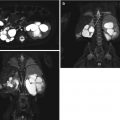
Fig. 18.2
DTPA renogram with furosemide at 20 min shows slow uptake and excretion bilaterally. The slope of the curve is flatter on the left. Does this mean obstruction?
The Whitaker Test
The classic Whitaker test combines a urodynamic study with an antegrade pyelogram to differentiate patients with residual or recurrent obstruction from nonobstructive dilation secondary to musculature changes. The Whitaker is performed by percutaneous puncture of the renal pelvis with or without placement of a nephrostomy tube. The upper tract is then perfused at rate of 5, 10, and 15 ml/min with saline or dilute contrast, while serial pressures are monitored in the renal pelvis and bladder. Obstructed systems will have a progressive rise in renal pelvis pressure above 12 cm H20, while nonobstructed systems will tolerate the increased volumes without rise in pressure [12]. Today, the Whitaker test is performed far less frequently than in the past, as other less invasive testing has become available for defining renal obstruction. The Whitaker antegrade pressure flow study is an invasive procedure with significant limitations, especially in infants whom normal data has not been established [13]. Still, the combination of a detailed antegrade pyelogram with direct pressure measurements can be helpful in problem cases (Fig. 18.3, for Whitaker study images on same infant presented in Figs. 18.1 and 18.2).
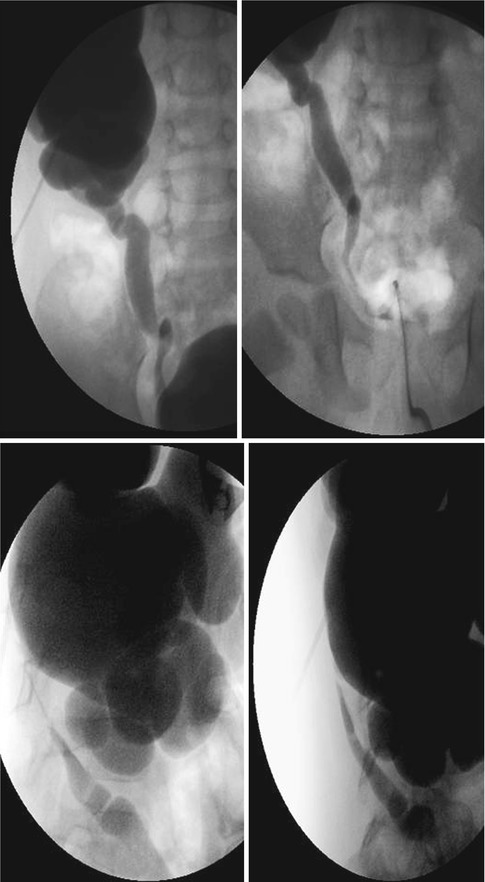

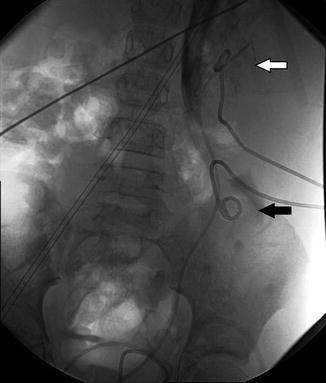
Fig. 18.3
AP and lateral images from antegrade pyelogram show tight narrowing at the UPJ. The Whittaker test showed an opening pressure of 17 cm of water. Following 10 min at 5 cc per minute, the pressure was approximately 35 cm of water. Following 10 min at 10 cc per minute, the pressure fell to 22 cm of water, and did not change at 15 cc per minute. This suggests significant but compensated left UPJ obstruction
MR Urography
MR urography has emerged over the past decade as an effective, noninvasive method for the evaluation of obstructive uropathy in the pediatric patient. MR urography combines excellent spatial resolution and high temporal resolution as it tracks the passage of contrast through the kidney giving both excellent anatomic and functional information in one exam without exposing the patient to ionizing radiation. Using a three-dimensional sequence to follow contrast through the kidneys, it is possible to calculate the renal transit time (RTT) which is defined as the amount of time between the appearance of contrast material in the kidney and its appearance in the ureter at or below the level of the lower pole of the kidney. A normal RTT is less than 245 s, and unequivocal obstruction is a RTT greater than 490 s. The left to right or upper to lower pole differential renal function can also be determined from the MR images with results comparable to renal scintigraphic studies. While the results between the two methods are comparable, MR urography has a significant advantage secondary to its greater resolution, contrast, and rendering of three-dimensional anatomic detail. The primary disadvantages of MR urography are its requirement for extensive post-processing and its susceptibility to motion, often requiring conscious sedation or even general anesthesia in the pediatric population in order to minimize the motion artifact. High costs and limited availability are other current disadvantages of MR utilization. However, its advantages still far exceed its limitations, and MRU utilization will likely continue to because of its unique combination of functional imaging and high-resolution detail without any ionizing radiation [14–17] (Fig. 18.4 is MR urogram for same infant discussed in Figs. 18.1, 18.2, and 18.3).
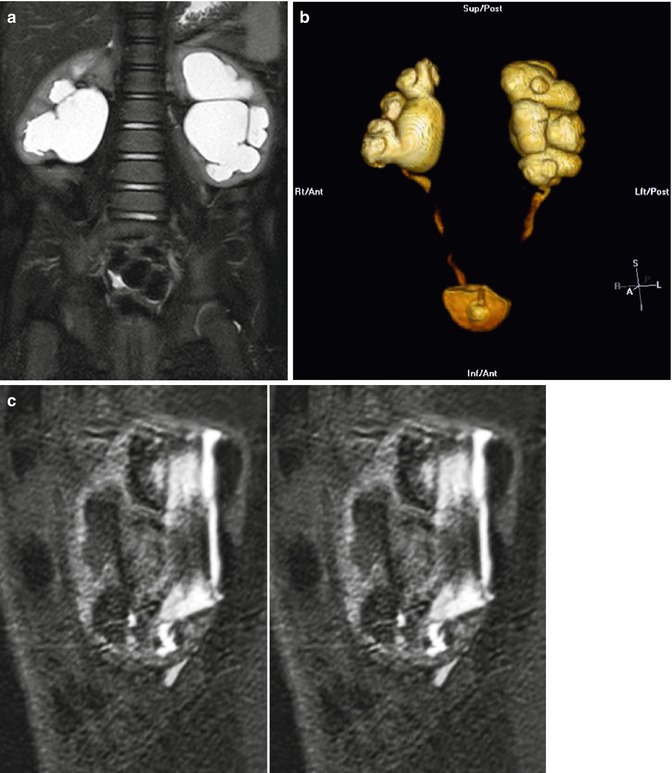

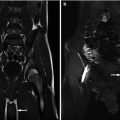
Fig. 18.4
Coronal T2 (a) and 3D (b) reconstructions show detail available on MRU. Thin slice lateral views of the left kidney show the narrowing of the ureter as it enters the pelvis, similar to the antegrade pyelogram (c)
Basic Techniques of Renal Access with Emphasis on the Child and Infant
The basic technique for renal access is the same in adults and children. In fact, it is much easier to visualize the infant kidney by US than in adults, because there is very little intervening soft tissue. Still, kidney sizes are very small, ranging from 25 mm in a premature infant to 50 mm in a full-term infant. Even a hydronephrotic collecting system may be only a cm or two in diameter. In addition, the kidneys are very mobile in the infant and young child, tending to move away from the needle or especially the dilator or catheter if you are not careful [18].
Just as in adults, it is not necessary for the patient to be completely asleep for a nephrostomy, just adequately sedated. Unlike adults who can cooperate if only partially asleep, most children and infants become frightened, agitated, and uncooperative when they are lightly sedated. As a result, moderate to deep sedation is required, and many anesthesiologists feel it is safer to incubate small children who are moderately sedated.
Needle Placement and Antegrade Pyelography
Once the patient is adequately sedated, they are positioned either prone (for bilateral nephrostomies) or partially on their side with the kidney needing access somewhat above the spine [19]. A preliminary US is helpful to locate the kidney and identify a safe access point and mark it on the skin. After standard sterile prep and drape, with the US in a sterile probe cover, after local anesthesia, an echo tip 22 G needle is advanced under US guidance into the kidney (Figs. 18.5 and 18.6). Like adults, use of a mid or upper pole calyx is recommended if ureteral access is planned. Generally, the needle can be well seen by US all the way into the kidney and calyx. If hydronephrosis is present, urine usually drips from the needle when the stylet is removed. A small amount of contrast is injected to demonstrate the collecting system and the position of the needle in the kidney. If necessary, the needle can be repositioned using US or fluoroscopy, or both [20]. Although an antegrade pyelogram can be performed with a needle only, we usually prefer to place a small catheter or dilator to provide more flexible and secure access.
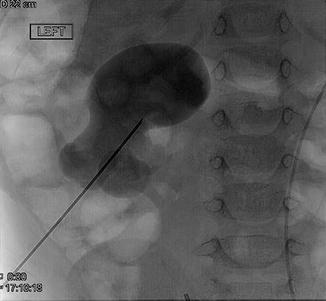
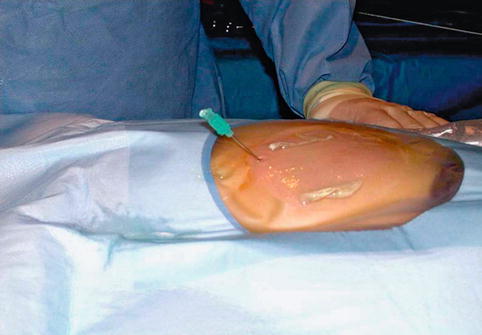

Fig. 18.5
Percutaneous access with needle to lower pole calyx is obtained

Fig. 18.6
Prone position of patient at time of percutaneous access is demonstrated. Ultrasound-guided access to kidney using micropuncture needle is obtained
Nephrostomy Tube Placement
Once the needle is in place, a 60 cm 0.018 in. guidewire can then be advanced into the renal pelvis and ideally down the ureter. Generally, a stainless steel mandril or nitinol wire can be positioned, with the stainless steel wire offering the greatest stiffness for subsequent dilation and catheter placement. Occasionally, a 0.018 in. hydrophilic guidewire (Glidewire) may be needed to either coil in the renal pelvis or maneuver into the ureter, but this wire is both too long (150–180 cm) and too flexible to easily allow advancement of dilators and the catheter. We routinely cut a Glidewire shorter, but this can leave a burr on the end making it harder to advance a dilator or catheter over the wire. Especially in infants, sequential dilation starting with a 4 Fr dilator is suggested, followed by a 5 or 6 Fr dilator or a “micropuncture” access kit allowing placement of a larger wire if needed, but larger wires are rarely helpful in small patients (Fig. 18.7).
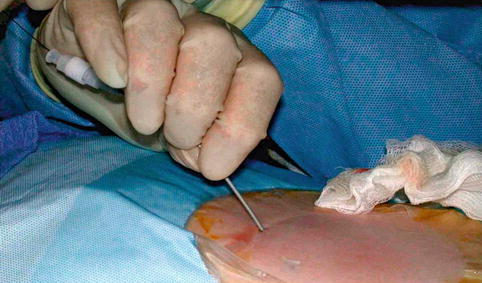

Fig. 18.7
Using Seldinger technique, dilator is passed over guidewire that was inserted through access needle
For infant nephrostomies, a 5 or 6 Fr drainage catheter is sufficient. Because the renal pelvis is small in an infant or child, use of a smaller loop catheter such as the Cook Dawson Mueller catheter is preferred. Hopefully, the renal pelvis is large enough to allow the catheter to form, but this can be problematic, especially if the wire cannot be advanced down the ureter or coiled in the renal pelvis [21]. This is where a Glidewire can help because it will usually coil more easily in the renal pelvis. Use caution if you use a Glidewire with the small drainage catheters because the plastic coating can be torn by the internal metal stiffener, making deploying the catheter difficult or impossible. The 5 Fr drain and stiffener requires a 0.018 in wire, the 6 Fr a 0.025in wire, and the 7 Fr a 0.032in wire. Generally, the 5 and 6 Fr catheters can be advanced over the 0.018in wire, while the 7 Fr will track better with a larger wire. This is where it becomes necessary to use a micropuncture access set to allow a larger wire. Still, we generally use only 5 or 6 Fr catheters in smaller patients.
The infant kidney can be pushed off the end of the guidewire by the dilator or catheter, especially if the guidewire could not be advanced down the ureter. Use of US to confirm that the dilators and catheter are inside the collecting system is frequently helpful because injecting contrast into a drain that is external to the collecting system makes it even harder to regain access [22]. In that situation, repuncture of the now decompressed collecting system under US is challenging but usually possible. Once the catheter is in place, the catheter is secured with one or two nylon sutures and a secure dressing and drainage bag applied.
Nephroureteral Stent Placement
Although infant-sized “double J” stents are now available, it is sometimes easier or desirable to place a nephroureteral stent. This allows external drainage for a time to treat infection or renal failure. Once infection or renal failure has improved, the external portion of the catheter can be capped to allow internal drainage. This has the advantage of relatively easy placement at the time of initial nephrostomy as compared to the antegrade placement of double J stents. In addition, it allows easy access for follow-up antegrade pyelograms to check ureteral stenosis or leak. Finally, it is easy to exchange these catheters should they become clogged, and it is usually easy to remove them at the completion of therapy.
Once renal access has been achieved, a guidewire is advanced down the ureter and hopefully into the bladder. If a UPJ or UVJ obstruction is present, or if there is marked ureteral tortuosity, use of an angiographic catheter and various guidewires may be needed. Once the wire is in the bladder, the UVJ, UPJ, or stricture can be dilated by angiographic catheter directly, or a coronary balloon advanced into position. Once the stricture is dilated as desired, the length of the ureter is measured by guidewire from the bladder to the renal pelvis. A standard drainage catheter is then modified with additional side holes measured so that they are within the kidney and ureter. This allows precise placement of the catheter so that excess catheter length in the bladder or kidney is not an issue. The modified catheter is then advanced over the wire into the bladder. Careful contrast injection to make sure all of the side holes are in the renal pelvis is essential. The catheter is secured in place and a drainage bag attached. Once external drainage is completed, the catheter is capped. If the patient exhibits signs of infection or obstruction after discharge from the hospital, the family can reopen the drain to external drainage at home. This allows trials of capping on an outpatient basis.
When removal is needed, it is preferred to remove catheters under fluoroscopy. Advance a guidewire down the catheter into the bladder to straighten the loop, and then remove the catheter and apply a dressing.
Antegrade Ureteral Stent Placement
Placement of a conventional double J (JJ) ureteral stent from above is also possible, although deployment of the catheter can be technically challenging, especially in a relatively non-dilated upper tract. Preferably, renal access is achieved into a mid or upper pole calyx. Placement of a small peel-away sheath helps to stabilize access to the kidney. A guidewire and catheter are advanced down the ureter and the ureteral length estimated with a guidewire. The conventional JJ stent can then be pushed down the guidewire into the bladder. Getting the proximal J to deploy properly can be challenging, and withdrawing the catheter back into the renal pelvis difficult, although the peel-away sheath allows the proximal string on the catheter to be withdrawn (Fig. 18.8). It is important to make sure the proximal end of the stent is not left in the renal parenchyma because pain and bleeding may occur. The nephrostomy tube can be left in for a day or two, especially in an infected system. Removal of the stent can be done from above, but is usually done from the bladder. Reliable estimation of a JJ stent length is often obtained by utilizing the formula of 10 + age of patient = length of JJ stent in cm [23].
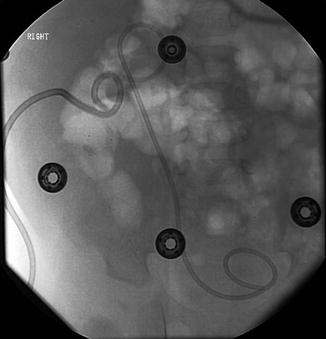

Fig. 18.8
Successful antegrade placement of double J stent via percutaneous renal access
Endopyelotomy/UPJ Balloon Dilation
Balloon dilation of the ureteropelvic junction in adults was first described in 1982 and is now well established and commonly performed using a retrograde approach by urologists. When retrograde catheterization of the ureter proves difficult, an antegrade approach via nephrostomy is employed. The complication rate of the procedure is low, and success rates up to 85 % have been reported [24]. Diagnosis of ureteropelvic junction obstruction in children is now frequently suspected on fetal ultrasound, but it may also remain undetected until infection is detected. Later presentations may include pain, hematuria, or stone formation. DTPA renal scan with furosemide assesses the degree of impairment of renal function. It can then be treated by surgical pyeloplasty. However, a minimally invasive technique by balloon dilation may be used to treat as well, either retrograde via nephrostomy or antegrade via cystoscopy. This technique is also commonly used to treat restenosis after pyeloplasty, especially when there has been more than one attempt at surgical correction [24].
Percutaneous Nephrolithotomy
The incidence of urolithiasis in pediatrics varies worldwide with geography and socioeconomic factors, but stones occur in children of all ages without gender predominance. Overall incidence of appears to be increasing globally, likely reflecting westernized lifestyle and dietary changes, including higher salt intake in processed food and decreased water consumption [25]. Anatomic abnormalities, urinary tract infections, and metabolic disturbances increase the risk for stone development, with hypercalciuria and hypocitraturia as most common metabolic risk factors [26].
Renal calculi in neonates and younger children are often diagnosed with ultrasound. Although location and presence of hydronephrosis can be accurately assess with this, 40 % of stones may be missed on ultrasound. Though computed tomography (CT) is more sensitive compared to ultrasound, concerns regarding radiation exposure limit its use in young children. Typically, asymptomatic calculi incidentally found are followed with serial US or plain films to minimize exposure. CT noncontrast is the diagnostic choice in older children, or younger children symptomatic with stone in which plain films or US are nondiagnostic [27].
Stones are treated conservatively, as long as pain is well controlled, the patient is tolerating liquids, and stone size is consistent with high chance of spontaneous passage. In cases of urinary tract infection, significant hydronephrosis with renal colic, or uncontrolled nausea and vomiting, a ureteral stent or nephrostomy may be placed to decompress the urinary system and allow resolution of edema before endourologic management is undertaken, usually 1–2 weeks later.
Percutaneous nephrolithotomy (PCNL) is indicated in children whose therapy with shock wave lithotripsy or ureteroscopy has failed and in those with anatomic abnormalities that impair urinary drainage and stone clearance, such as ureteropelvic junction obstruction, ureteroenteric anastomosis, previous ureterovesical surgery, infundibular stenosis, and caliceal diverticulae [28]. Special considerations in children include preserving renal development and function, limitation of radiation exposure, and minimizing need for retreatment and, if the latter does become necessary in the future, to not limit or preclude any possible treatment options [29].
The first PCNL series was reported in 1985 by Woodside et al. and included seven children with a mean age of 14 years, treated using instruments for adults with no significant complications [30]. Unsal et al. compared the morbidity and success differences in use of adult versus pediatric-sized devices for PCNL for two groups of children, those less than 7 and greater than 7 years old. Pediatric instruments were used in preschool children and children without dilated collecting systems and small stone burden, and adult instruments and techniques in older children with dilated collecting systems achieved equal results. There was less postoperative bleeding if the degree of dilation was less. Small instrument size did not increase the operative time and resulted in the same success rates as adult-sized devices [31].
Over time, many standardized adult stone treatment options have proven efficacious and safe in treating children. With age no longer a limiting risk factor, recent reports have shown that almost any version of PCNL can be applied safely in children as well. The Clinical Research Office of the Endourological Society (CROES) presented retrospective data review of 24 centers treating children under age of 14 years old, demonstrating the differences between pediatric age groups (<1 year old infants, 1–4 years old preschool children and 5–14 years old school age children) and adults in the indications, complications, and outcomes of treatment with PCNL. The preferred method for determining postoperative stone-free status was ultrasound in preschool children over KUB abdominal x-ray in school age children and adults (>15 years old). Although there were no significant differences between the age subgroups for operative details, the mean sheath size and nephrostomy tube size were larger in school age children compared to younger subgroups [32].
PCNL procedure is performed under general anesthesia, with antibiotic prophylaxis after preoperative urinary cultures demonstrate sterile urine. The patient is initially placed in the lithotomy position for stent or catheter placement, and a retrograde pyelogram is performed to outline the collecting system. The patient is then repositioned in the prone position. The desire calyx is the selected, either by ultrasound guidance or under fluoroscopy using biplane orientation—the ideal tract provides the shortest most direct access to the stone. After access is confirmed, a flexible guidewire is then placed into the collecting system and directed down the ureter into the bladder. A small skin incision is made with a No. 11 blade, and then coaxial dilators, 8F and 10F, are passed into the collecting system. Next an Amplatz Super Stiff guidewire is placed as the working wire, the previous wire clamped to the drape and saved as safety wire. Serial dilators, a balloon dilator, or a small peel-away sheath and trocar are placed over the wire into the calyx under fluoroscopic guidance (Figs. 18.9 and 18.10). Once adequate dilation has been achieved, depending on the size of the sheath, rigid or flexible nephroureteroscopes can be employed to identify the stone, and working ports within the scopes are used to pass either lasers or lithotripters to break up the stones. Fragments are then removed by irrigation or stone basketing. The outer diameter of nephroscopes range from 17 to 26F, and flexible nephroscopes are 15F with 6F working channel. Offset cystoscopes 7 and 8F with 5F working ports can also be used. Flexible ureteroscopes 7–9F can be used through 11 F sheath, with enough clearance to allow low-pressure irrigation. Electrohydraulic, ultrasonic, and pneumatic lithotripsy, as well as holmium laser lithotripsy, is effective for stone fragmentation. Finally, after the stone has been adequately treated, postoperative drainage of collecting system with stenting (“tubeless”) or placement of a nephrostomy tube will be performed, with imaging assessment to follow [30].
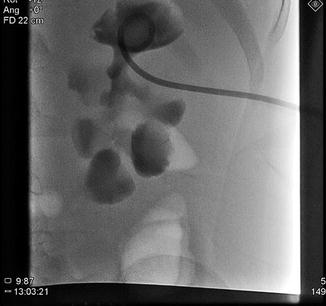
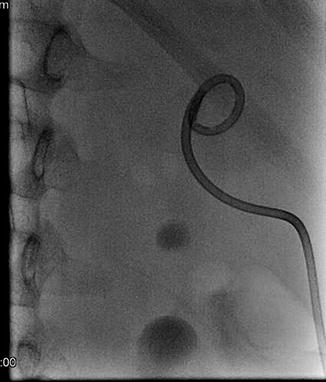

Fig. 18.9
Percutaneous access, in preparation for percutaneous nephrolithotomy (PCNL) is obtained into upper pole calyx with antegrade pyelography. A 1.5 cm renal pelvis stone is seen (More visible in Fig. 18.10)

Fig. 18.10
A 1.5 cm renal stone is seen in position of renal pelvis while successful access to upper poly calyx already accomplished as in Fig. 18.9
Mini-perc is a novel percutaneous renal access technique developed using an 11F peel-away vascular access sheath, the system passed over a single wire. This was developed in an effort to decrease the morbidity of percutaneous nephrolithotomy, hoping to minimize blood loss and damage to nephrons. In the Jackman study, seven renal units underwent the “mini-perc” procedure, with three take backs for second look resulting in overall stone-free rate of 85 %. No transfusion or urosepsis complications were reported. 7F rigid pediatric cystoscope or 9F flexible ureteroscope was used in most cases, and stones fragmented with electrohydraulic lithotripsy probes [33].
Residual stone fragments are associated with adverse clinical outcome, and every attempt should be made to achieve a stone-free status. In effort to reduce the number of tracts and associated morbidity, some centers choose to follow primary PCNL with adjunctive shock wave lithotripsy (SWL) to clear residual fragments. After SWL sandwich therapy, the stone-free rate increases to 100 % [29]. Second-look nephroscopy through the original tract to ensure stone-free status is another alternative, done at the same hospital admission rather than another admission and procedure.
Percutaneous Cystostomy
Direct percutaneous bladder drainage is rarely needed in children. Bladder outlet obstruction is usually due to congenital urethral obstruction from posterior, or less commonly anterior, urethral valves. This is generally managed by cystoscopic fulguration of the valves. If the bladder needs short-term drainage, a Foley catheter is used. For longer-term decompression, cystostomy can be performed. The technique is similar to nephrostomy placement or abscess drainage from an anterior suprapubic approach.
Retroperitoneal Interventions
Renal and Perirenal Abscesses and Fluid Collections
Perirenal fluid collections usually resolve spontaneously, but when they become infected or are producing significant mass effect, percutaneous drainage can be helpful. Guidance is almost always by US, although CT can be helpful in larger patients. The basic technique is the same as the nephrostomy tube placement and is generally easier since the collection is usually larger than the collecting system and the point of needle access less critical. Care needs to be taken to avoid adjoining bowel (usually the ascending or descending colon). Once the needle is in place, a sample of the fluid is obtained before contrast injection if possible. A guidewire is passed into the collection and the tract dilated. Finally, a suitable-size drainage catheter is placed. If the fluid is simple, such as a urinoma or lymphocele, a relatively small catheter is appropriate. If it is infected or hemorrhagic, catheters of at least 10 Fr should be placed (Fig. 18.11). An attempt to aspirate as much of the fluid as possible should be performed, confirming placement of catheter with US and fluoroscopy. Occasionally, it is necessary to maneuver the catheter into a better position to assure complete drainage. The catheter is sutured in place and a drainage system applied. Postoperative lymphoceles may require sclerotherapy to prevent recurrence. Agents such as doxycycline or absolute alcohol can be used. If a perinephric urine collection continues to drain, placement of a nephrostomy or stent may be needed to control the leak from the renal collecting system. In infected spaces, drainage catheters are usually left in place until the drainage is clear and less than 10–20 cc/day. Irrigation of catheters left in infected spaces to assure patency with 5–10 cc of normal saline 2–4 times daily is recommended.