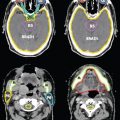
FIGURE 19-10a. CTV delineation in a 72 year-old patient receiving definitive IMRT treatment for prostate cancer. Bladder is yellow, rectum is orange, femoral heads are magenta, and CTV is red.
1. ANATOMY
• The prostate is bounded superiorly by the bladder, inferiorly by the urogenital diaphragm, anteriorly by the pubic symphysis, and posteriorly by Denonvilliers prostatic fascia and the rectum.
• The urethra courses from the bladder neck superiorly to the prostatic urethra and through the urogenital diaphragm, bulbous urethra and penile urethra antero-inferiorly.
• A sagittal view of the prostate is shown in Figure 19-1.
• The prostate is triangular in shape, with the base situated superiorly and the apex inferiorly.
• A capsule encases the prostate except at the apex of the gland, where the prostate blends into the urogenital diaphragm.
1.1. Zonal Anatomy
• The glandular prostate is divided into three anatomical zones (Fig. 19-2).1 The peripheral zone, located posteriorly, is the largest, comprising 70% of the glandular tissue. About 75% of prostatic malignancies occur in the peripheral zone.
• The transition zone surrounds the urethra in the mid-prostate and tends to enlarge with age as a consequence of benign prostatic hypertrophy (BPH), causing urinary obstructive symptoms. The transition zone comprises 5% to 10% of the glandular tissue and 20% of malignancies.
• The central zone, located superiorly, contains about 20% of the glandular tissue, but only 5% of the prostatic malignancies. The ejaculatory ducts pass through the central zone and empty into the urethra at the verumontanum.
• The anterior fibromuscular stroma has very little glandular tissue and malignancies rarely originate in this region.
1.2. Erectile Tissues
• Erectile function is controlled mainly by the neurovascular bundles, which are located posterolaterally to the prostate.2–4
• Radiation dose to the penile bulb and corporal bodies has been suggested as a determinant of erectile function.5,6
1.3. Seminal Vesicles
• The seminal vesicles are located superior and posterior to the base of the prostate.
• Invasion of the seminal vesicles is a relatively common pattern of spread for intermediate and high-risk prostate cancer.7,8
2. NATURAL HISTORY
2.1. Lymphatics
• Lymphatic spread has been related to pretreatment prostate-specific antigen (PSA), Gleason score, and stage.9–14
• The most common sites of involvement are the obturator, external iliac, and internal iliac lymph nodes. The presacral lymph nodes are also at risk.15,16
• Lymph node dissection prior to radical prostatectomy involves an en bloc resection of the fatty tissue from the external iliac vein medially to the obturator vessels and nerve, extending inferiorly from the inguinal ligament to the bifurcation of the common iliac vessels superiorly.
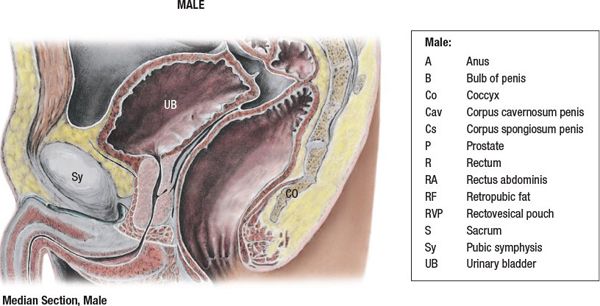
FIGURE 19-1. Medial section through the male pelvis. (Reprinted from Agur AMR, Dalley AF. Grant’s Atlas of Anatomy, 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:279.)

FIGURE 19-2. Structure of prostate gland. (From Anatomical Chart Company, copyright © 2008, Lippincott Williams & Wilkins. All rights reserved.)
2.2. Metastasis
• Lymph node metastasis is typically <15% in the absence of T3 disease13; however, high rates may be seen in T1/T2 tumors when the Gleason score is 8 to 10 and/or the PSA is >20 ng/mL.11,17 The Roach formula has been used to estimate lymph node risk in patients with T1/T2 disease utilizing only two variables, the pretreatment PSA, and the Gleason score.18,19
• Hematogenous metastases are typical to the axial skeleton (vertebral bodies and ribs).
• Soft tissue metastases, such as to the liver, lung, or brain, can rarely occur late in the disease course.
3. DIAGNOSIS AND STAGING SYSTEM
3.1. Signs and Symptoms
• Many patients with prostate cancer present with urinary obstructive symptoms. In most cases, this is due to BPH, which is common in elderly men.
• An assessment of urinary function using the International Prostate Symptom Score (IPSS) questionnaire is a useful tool for determining whether patients will have significantly more acute urinary morbidity with a seed implant compared with external beam radiotherapy (EBRT) (IPSS > 8 to 10) or whether they should be considered for a transurethral resection of the prostate prior to EBRT (IPSS > 20).20–24
• Diagnosis is usually made by ultrasound-guided prostate biopsies provoked by either a change in the rate of increase in PSA (velocity > 0.75 ng/mL per year), a high absolute PSA (>4.0 ng/mL), and/or a low free to total PSA (<25% when the absolute PSA is greater than 4.0 to 10 ng/mL).25–27
• When high-risk features are present (a positive family history or African American descent),28,29 biopsies may be recommended at lower absolute PSAs (>2.5 ng/mL).30–33
• Bone pain is a sign of metastatic disease.
3.2. Physical Examination
• Digital rectal exam (DRE) is fundamental to the staging of prostate cancer.
3.3. Imaging
• Bone scan and computed tomography (CT) scan of the pelvis are recommended when high-risk features are present (palpable T3 disease, Gleason score of 8 to 10, or a pretreatment PSA > 20 ng/mL).34,35 Although the yield is low, some advocate a bone scan if the PSA is >10 ng/mL.35
• Endorectal coil magnetic resonance imaging (MRI) has been advocated as a routine test for identifying extraprostatic extension.36–39 We have used it when DRE reveals bulky disease, suspicious for extraprostatic extension. If obvious extraprostatic extension is identified in this setting, androgen deprivation would then be recommended for the patient. We have also used it for patients with limited intermediate-risk features who are adamant about undergoing a seed implant.
• Because the consistency of the Prostascint scan has been criticized, it is not routinely used to evaluate for metastatic disease.40,41
• Positron emission tomography (PET) scanning has not been proven to contribute to prostate cancer staging as an additional modality to the methods described above.40,42
3.4. Staging
• The 7th edition of the American Joint Committee on Cancer (AJCC) staging system for adenocarcinoma of the prostate, published in 2010, detailed a small number of changes from the 6th edition.43
• The clinical T-staging system is primarily based on the findings of the DRE. The updated staging system modifies the stage groupings by taking into account the PSA and Gleason score.
• Although imaging studies, such as ultrasound and MRI, and laterality findings at biopsy are informative for disease assessment, the information provided by these has not been consistently applied.44 The inclusion of imaging and biopsy results has resulted in stage migration and they have not adequately been documented to predict for outcome independent of the DRE. A T2a on DRE may be upstaged to a T2b or T2c based on ultrasound or biopsy findings. Thus, the gold standard for tumor staging is the DRE.
• As mentioned above, endorectal MRI has proven to be promising in the categorization of extraprostatic extension and for supplementing the DRE.36,37,45 The use of MRI for the evaluation of prostate cancer is increasing, however, it is not yet considered a routine part of the workup.
• If imaging is included in the staging, it should be documented.
4. ENDPOINTS
• Perhaps the most significant endpoint used is freedom from a rising PSA or freedom from biochemical failure.
• Biochemical failure is strongly related to local failure as well as distant metastasis and cause-specific death.46–50. The relationship of biochemical failure to overall mortality is still not well defined.48
• The Phoenix definition for biochemical failure after EBRT, with or without androgen deprivation therapy, is currently the accepted definition. It defines failure as a rise in PSA of 2 ng/mL above the nadir.51,52
5. PROGNOSTIC FACTORS
5.1. T-Stage
• When patients with distant metastasis and lymph node involvement are excluded, T-stage is a significant predictor of biochemical, local, and distant failure.53–55
5.2. Prostate-Specific Antigen
• Pretreatment prostate-specific antigen (PSA) is the strongest predictor of biochemical failure and is also significantly associated with local and distant failure, cause-specific death, and overall mortality.46,54–56
5.3. Gleason Score
• Adenocarcinoma is the most common histology which is seen in over 95% of cases of prostate cancer.
• The Gleason scoring system is the most frequently used grading system. Major and minor patterns, ranging from 1 to 5, are assigned based on the glandular pattern. The total score is the sum of the major and minor glandular patterns, which ranges from 2 to 10. The higher scores predict for more aggressive disease.57
• The Gleason score is a strong predictor of distant metastasis and survival.54,55,57
5.4. Predictive Models
• A number of models have been advanced for prediction of outcome after definitive treatment.58,59 The National Comprehensive Cancer Network (NCCN) has adopted a similar model to that delineated in Table 19-1 to allow basic stratification of patients without metastases into risk groups. These groupings provide a means of quickly estimating the risk of biochemical failure.
• Other predictive models such as the Partin tables60 allow a more individualized approach to a particular patient. This is particularly true in patients where all the risk factors do not conform to a particular group, such as with high Gleason score, but low PSA.
6. GENERAL MANAGEMENT
• Expectant management (i.e., watchful waiting) is a treatment option for elderly patients with a low likelihood of living 10–15 more years due to comorbid conditions. Patients who underwent expectant management died of prostate cancer in 18% to 30%, 42% to 70%, and 60% to 87% of cases when their Gleason scores were 6, 7, or 8 to 10, respectively.61–63
• When undergoing curative therapy, patients at New York Presbyterian Hospital—Columbia University Medical Center (NYPH-CUMC) are stratified into risk groups based on the T-stage, pretreatment PSA, and Gleason score (Table 19-1).
• Radical prostatectomy and radiotherapy offer comparable control rates for patients with favorable risk features (PSA <10 ng/mL, Gleason score <7, and T1/T2 disease), and for most patients with intermediate-risk features (PSA 10 to 20 ng/mL or Gleason score 7 in the absence of high-risk features).64 However, there are no contemporary randomized studies to support this assumption.
• When intermediate features are present, low dose rate brachytherapy as monotherapy may have inferior results compared with EBRT or surgery.65,66
• When high-risk features are present (T3/T4 disease, PSA >20 or Gleason score 8 to 10), EBRT is often recommended due to the high likelihood of positive surgical margins, extracapsular disease, seminal vesicle invasion, and/or lymph node involvement, which would necessitate postoperative radiotherapy.67
• Long-term (2–3 years) androgen deprivation is combined with EBRT when any high-risk feature is present.57,68–71
7. INTENSITY-MODULATED RADIATION THERAPY FOR PROSTATE CANCER
7.1. Target Volume Determination
• Gross tumor volume (GTV) for adenocarcinoma of the prostate is not visualized well and therefore is not contoured separately. Some investigators use functional imaging to distinguish a GTV for dose escalation with magnetic resonance spectroscopy72–74 or Prostascint scans,75 but these are clearly investigational. Also investigational is the use of focal therapy to part of the prostate.76
• The clinical target volume (CTV) is determined by the patient’s respective risk group (low, intermediate, or high), which includes the prostate with varying amounts of the seminal vesicles (Table 19-2).
• The CTV for low-risk disease includes the prostate ± the proximal seminal vesicles.77,78 The CTV for high-risk disease covers the prostate and entire seminal vesicles as outlined in Table 19-2.
• The coverage of pelvic lymph node stations should be considered in the CTV for high-risk patients. The target volume specification for definitive IMRT in low-, intermediate-, and high-risk prostate cancer is summarized in Table 19-2.
7.2. Target Volume Delineation
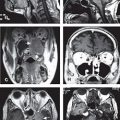
• Using CT simulation alone, the volume of the prostate may be overestimated by 30% to 40% due to difficulty in precisely determining the apex, base, and radial borders of the prostate (Fig. 19-3).79,80 It is reasonable to consider using an MRI for challenging cases to help delineate the target accurately.
• It is helpful to review the article by McLaughlin et al.81 to gain a better understanding of prostate anatomy on CT, and how to avoid common pitfalls. Useful information is also available at http://www.prostadoodle.com/.
• The seminal vesicles are contoured as described in Table 19-2.
7.3. Planning Target Volume Determination
• The planning target volume (PTV) is the added margin, which takes into account all the uncertainties in target position, including daily patient setup and positional changes due to rectal filling, bladder filling, and respiration during treatment.82–86

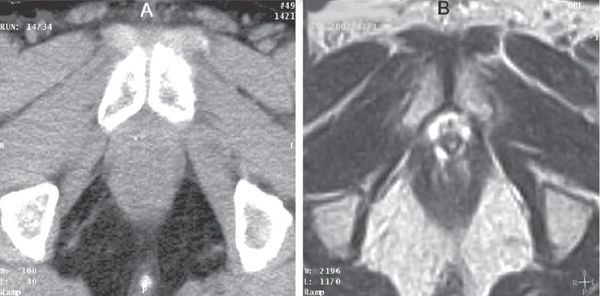
FIGURE 19-3. Corresponding axial computed tomography (CT; at left) and magnetic resonance (MR; at right) images of the prostate apex demonstrate a clear benefit at delineating the apex and the rectal–prostate interface.
• Interfractional motion requires an additional 1.1 cm margin to ensure that the CTV is within the PTV 95% of the time.85 In order to reduce this uncertainty, the prostate must be immobilized87 (i.e., daily rectal balloon) and/or localized every day using implantable fiducial with portal imaging,88–91 daily transabdominal ultrasounds,92 or daily CT scans in the treatment room.93 Displacement of the prostate during radiotherapy (intrafraction motion) is minimal,90,93,94 although it can be increased in the prone position.83,88,95
• The absolute PTV margin used at NYPH-CUMC is 5 mm posteriorly and 7 mm in all other dimensions. These tight margins can be used because the patients undergo daily pretreatment imaging using the onboard kilovoltage CT imaging on the Varian Trilogy linear accelerator (Varian Medical Systems, Palo Alto, CA) to correct for interfractional motion.96
• Patients are also treated after daily placement of a rectal balloon (Radiadyne, Houston, TX). This has been shown to immobilize the prostate, as well as to allow reduction of the dose and toxicity to the rectum.97,98
• Patients are simulated and treated with a full bladder. This allows reduction of the dose to the bladder. It also helps to raise the bowel out of the radiation field.
7.4. Dose Specification
• Target delineations for two patients are shown in Figures 19-4 and 19-5. Figure 19-4 is for a patient receiving definitive IMRT, and Figure 19-5 is the IMRT plan for postoperative irradiation of the prostate bed.
• There are several sequential dose-escalation trials in the PSA era that have demonstrated a dose–response for patients treated with three-dimensional conformal radiotherapy (3DCRT).59,99–103 There is some evidence of benefit for all risk groups.
• These findings are echoed by the prospective randomized trial published by the M.D. Anderson Cancer Center, which demonstrated a significant benefit in freedom from failure for patients with a pretreatment PSA >10 ng/mL treated when treated to 78 Gy as compared with 70 Gy (Fig. 19-6).99,104 In this trial, the dose was prescribed to the isocenter.
• The dose used for definitive IMRT for prostate cancer at NYPH-CUMC is 81 Gy (Table 19-2). The prescription ensures that at least 91% of the PTV (D91) receives the prescription dose.
7.5. Normal Tissue Delineation
• At NYPH-CUMC, we generally follow the recently published Pelvic Normal Tissue Guidelines for Radiation Therapy.105
• The entire circumference of the rectum is outlined from the ischial tuberosities to the point at which the rectum loses its round shape and begins meeting the sigmoid flexure. The patients are given a bowel preparation the evening prior to the simulation, and an enema on the morning of the simulation, which gives a worst-case scenario in regard to planning and evaluating the dose–volume histogram (DVH) of the rectum.
• At NYPH-CUMC, patients are treated with a full bladder, as detailed above. The entire circumference of the bladder is contoured from its base through the dome.
• Bowel is contoured using the bowel bag approach, as detailed in the consensus guidelines.
• Currently, no effort is made to contour the urethra, neurovascular bundle, or penile bulb.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree