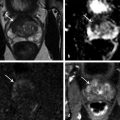
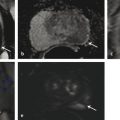
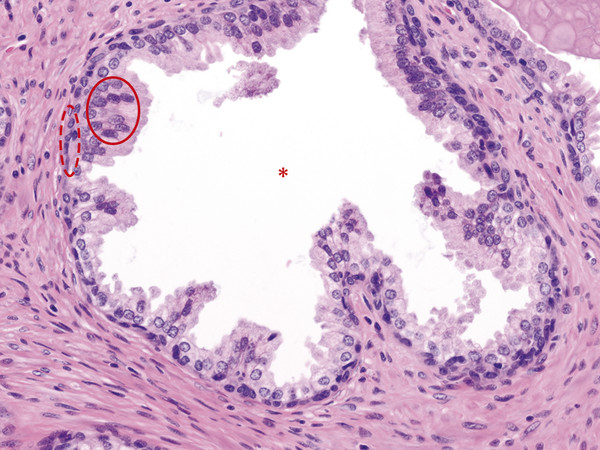
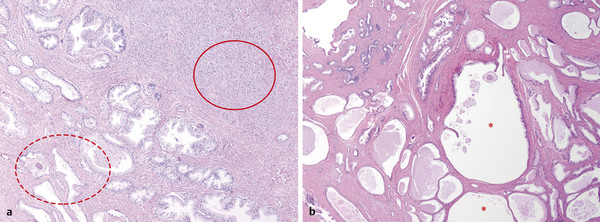
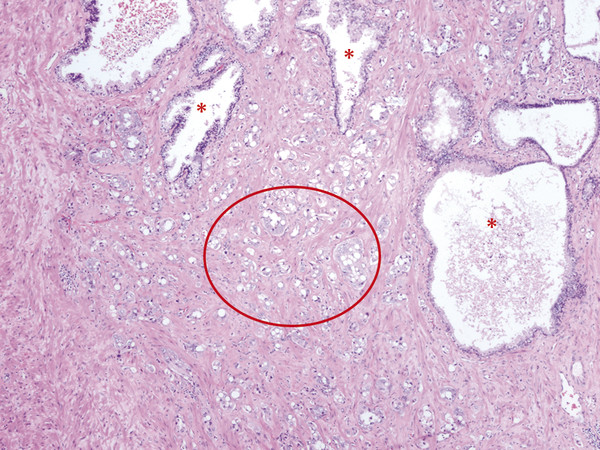
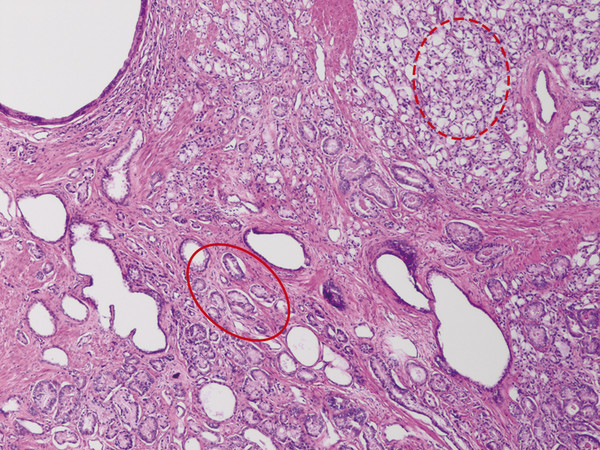
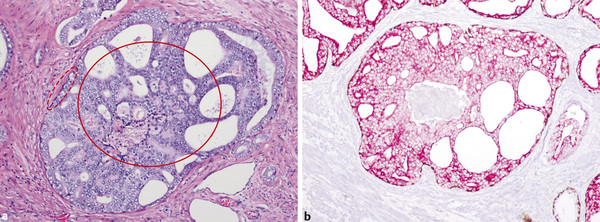
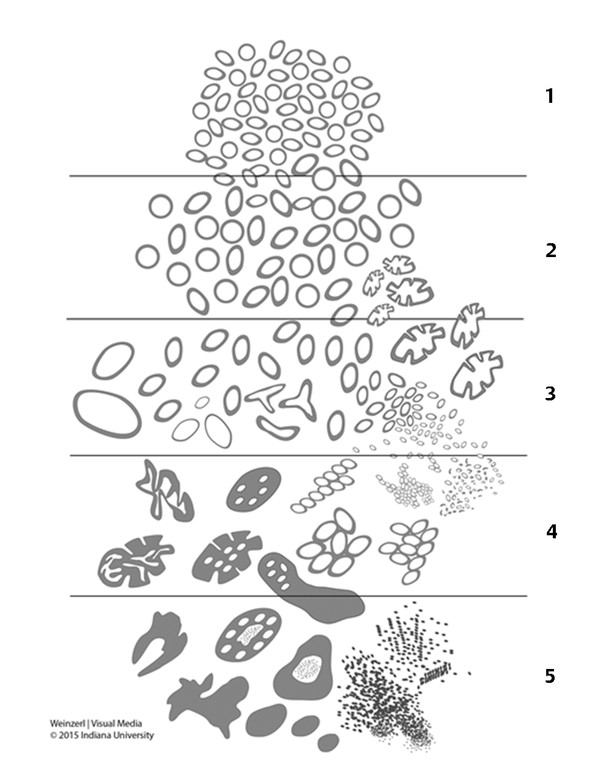
In an adult male without significant hyperplasia, the average weight of the prostate gland is approximately 20 to 30 g. It is shaped as an inverted cone, with the base at the bladder neck and the apex at the urogenital diaphragm. Anatomically and biologically, the prostate can be divided into three glandular zones (peripheral, central, and transition zones) and a fourth nonglandular region, termed the anterior fibromuscular stroma. The central zone (~ 25% of the prostate volume) is an inverted cone structure with ducts branching from the verumontanum to the prostate base and surrounding the ejaculatory ducts. The transition zone (5% of the prostate volume) lies in the bilateral base to midregion of the gland and is composed of ducts that extend laterally from the urethral wall and curve anteromedially. It typically enlarges in older men due to benign prostatic hyperplasia. The peripheral zone (70% of the prostate volume) extends postero-laterally around the central zone and distal prostatic urethra. 1, 2 Histologically, the prostate consists of epithelial and stromal cells. Epithelial cells are arranged in tubuloalveolar glands that consist of ducts that branch out from the urethra and terminate in acini. The glands have an irregular contour with luminal undulation and papillary infolding. The glands comprise mainly two cell types: luminal secretory cells and basal cells. Secretory cells have a columnar or cuboidal shape with clear to pale cytoplasm and pseudostratified nuclei. Basal cells are small, flat, and situated at the periphery of the glands beneath the secretory cells ( ▶ Fig. 2.1). Central zone glands are larger than peripheral and transition zone glands and more complex, with intraluminal ridges, papillary infolding, and occasional epithelial arches and cribiform glands that mimic prostatic intraepithelial neoplasm. The acini are mainly lined by luminal secretory cells and basal cells. The proximal portion of the prostatic duct is lined by urothelial cells. The distal portion of the prostatic ducts, as well as some acini, may exhibit cuboidal and columnar epithelium admixed with urothelium. Fig. 2.1 A normal prostate gland exhibiting irregular contour with luminal papillary folding. It comprises mainly two cell types: luminal secretory cells (solid oval) and basal cells (dashed oval). Secretory cells are columnar or cuboidal shaped with clear to pale cytoplasm and pseudostratified nuclei. Basal cells are small, flat, and situated at the periphery of the gland beneath the secretory cells. asterisk, glandular lumen. Benign prostatic hyperplasia (BPH), also known as nodular hyperplasia, is a common urologic condition related to overgrowth of the epithelium and fibromuscular tissue of the transition zone and periurethral area. Grossly, nodular hyperplasia consists of variably sized nodules that are rubbery, firm or soft, and yellow-gray, with a bulging surface. Nodular hyperplasia is composed of varying proportions of epithelium and stroma (smooth muscle and fibroconnective tissue). The glandular component of BPH is made up of hyperplastic small and large acini and often shows cystic change ( ▶ Fig. 2.2 a; ▶ Fig. 2.2 b). The luminal secretory epithelium consists of tall columnar cells with pale-staining cytoplasm. The basal cells are variably seen, ranging from barely detectable to hyperplastic. Fig. 2.2 Benign prostatic hyperplasia (BPH) (a), Stromal (right upper solid circle) and glandular proliferation (left lower dashed circle). (b), Cystic dilated glands (asterisk) in a BPH nodule. Prostate cancer is the most common noncutaneous cancer in American man, with increasing incidence in older age groups. The American Cancer Society estimates about 220,800 new cases of prostate cancer and about 27,540 deaths from prostate cancer in 2015. While about 6 cases in 10 are diagnosed in men aged 65 or older, it is rare before age 40. The average age at the time of diagnosis is 66. Prostate cancer is the second leading cause of cancer death in American men, behind only lung cancer. About 1 man in 38 will die of prostate cancer. 3 Most prostate cancers arise in the peripheral zone, and some can result in abnormal findings on digital rectal examination. Histologically, prostate carcinoma has a constellation of architectural, cytoplasmic, nuclear, and intraluminal features. Architecturally, the glands of gland-forming prostate carcinomas are more crowded than normal and typically exhibit a haphazard growth pattern, with these malignant glands oriented perpendicular to each other and irregularly separated by fibromuscular bundles. They also display infiltrated growth pattern, with malignant glands situated between or flanking benign glands ( ▶ Fig. 2.3). When prostate carcinoma becomes less differentiated, it partially or totally loses glandular differentiation and forms cribiform structures, fused glands, poorly delineated glands, solid sheets or cords, and even single cells ( ▶ Fig. 2.4). Prostate carcinoma typically displays enlarged nuclei with prominent nucleoli. Mitoses and apoptotic bodies are not frequently seen in prostate adenocarcinoma but are more common in this setting than in benign glands. Fig. 2.3 Prostate adenocarcinoma glands display infiltrative growth pattern, with malignant glands (for example, circle) situated between or flanking benign glands (asterisks). Fig. 2.4 Well-differentiated prostate adenocarcinoma is gland-forming. The malignant glands display more crowded architecture than normal glands, and typically exhibit a haphazard growth pattern (solid circle, lower half). Poorly differentiated prostate adenocarcinoma partially or totally loses glandular differentiation and forms cribiform structures fused glands, poorly delineated glands, solid sheets or cords, and even single cells (dashed circle, upper half). It is well documented that prostate cancer (PCa) presents as a multifocal disease, with two or more tumor nodules present within the prostate gland, in the majority of cases. 4, 5, 6, 7 Prostate cancer also demonstrates heterogeneity among different tumor nodules in the same prostate gland. Histologically, different tumor nodules in the same prostatectomy specimen often show different Gleason scores. 5, 8 Arora et al showed multifocal cancer was present in 87% of radical prostatectomy (RP) specimens. However, in only 9% of the cases of multifocal cancer did all tumor nodules have the same primary and secondary Gleason grades as the overall Gleason grades assigned to the RP specimen. 5 At molecular and genetic levels, Cheng et al studied the pattern of allelic loss in prostate cancer from patients who had two or more separate cancer foci and observed that the pattern of allelic loss was distinct between different foci in 15 of 18 cases, supporting an independent clonal origin of the multiple tumor foci in a single patient. 9 A recent study on TMPRSS2 gene rearrangements in multifocal PCa demonstrated differing gene arrangement status and class between different tumor foci, providing further molecular evidence of independent clonal origin of multifocal cancer foci. 10 The morphological and genetic heterogeneity of multifocal PCa suggests that different cancer foci may be biologically distinct, with the presumption that some tumor foci are more aggressive than others within the same prostate gland. The concept of the dominant nodule (DN) was first introduced by McNeal et al 11 to refer to the tumor nodule that most likely harbors the most aggressive biological behavior among the multifocal tumor nodules within the prostate and therefore presumably dictates the tumor’s overall biological behavior. In 2005, the International Society of Urological Pathology (ISUP) consensus recommended use of the DN for tumor grading and tissue banking for research on RP specimens. 12 The DN concept has also garnered considerable interest recently in focal therapy for PCa, as the DN is naturally the ideal target for therapeutic intervention. However, the definition of the DN is ambiguous in terms of which of the pathologic parameters (tumor size, Gleason grade [GG], or staging parameter) should be used to in fact determine which nodule is the DN. At the 2009 ISUP consensus meeting, the urologic pathology experts did not reach a consensus on the pathologic parameters that define the DN in RP specimens. 13 Currently, the DN is generally defined as the tumor nodule of the largest size in the setting of multifocal disease. 5, 6, 7, 11, 12 However, the largest tumor volume, highest GS, and staging parameters (i.e., extraprostatic extension) do not always occur in the same tumor nodule. 5, 7 Our group’s study 14 showed that prognostically important pathologic parameters (largest tumor volume, highest GS, and staging parameters) occur in the same tumor nodule in the majority of multifocal prostate cancer (88.7%), such that the concept of the DN is valid in these patients. In these cases, the DN can be used to assign an overall GS and procure tissue for research. However, adverse pathologic parameters (largest tumor volume, highest GS, and staging parameters) did not occur in the same tumor nodule in 11.3% of cases. In these cases, pathologists may deemphasize the DN concept and instead report the multifocality and pathologic features of all independent tumor foci. The notion of insignificant PCa has progressively emerged in the last two decades. The clinical relevance of such a definition was based on previous studies that suggested that low-grade, small volume, and organ-confined PCa may be indolent and unlikely to progress to biological significance in the absence of treatment. 15, 16 An accurate definition of insignificant disease may be important for the clinician to better manage patients after radical treatment and propose alternative therapies (active surveillance) more confidently. To date, the most commonly used criteria for defining insignificant PCa are based on the pathologic assessment of the RP specimen and include three well-established prognostic factors: (1) Gleason score (GS) no more than 6 without any Gleason pattern 4 or 5, even as a tertiary Gleason pattern; (2) organ-confined disease (no extraprostatic extension [EPE], seminal vesicle invasion [SVI], and lymph node invasion [LNI]); and (3) tumor volume < 0.5 cm3. The pathologic criteria for insignificant PCa might be improved by incorporating factors other than the pathologic features alone, such as age, prostate-specific antigen level (PSA) level, and comorbidities. 17 The differentiation of patients with significant versus insignificant PCa based on prostate biopsy may be more important. Men harboring insignificant PCa can be selected for active surveillance, while those with significant PCa usually require definitive treatment such as prostatectomy. The most commonly used biopsy pathology criteria for insignificant PCa is the Epstein criteria: no Gleason pattern 4 or 5, less than 3 cores from a sextant biopsy positive for tumor, and no core with > 50% tumor involvement. 18 However, minimal disease on biopsy does not reliably predict minimal disease in the subsequent prostatectomy specimen in terms of the size and grade of tumor, extraprostatic extension, or positive margins. Thus, reasoned accounting should be made of other data, particularly prostate-specific–antigen kinetics and potential molecular markers, before undertaking a course of active surveillance or radiation therapy as monotherapy. 19, 20 Prostate cancer is typically composed of usual acinar adenocarcinoma with a minority of acinar carcinoma and non–acinar carcinoma variants or types. Variants of usual acinar adenocarcinoma defined in 2004 by the World Health Organization (WHO) include atrophic, pseudohyperplastic, foamy, mucinous (colloid), signet ring, oncocytic, and lymphoepithelioma-like carcinomas ( ▶ Table 2.1). Adenocarcinomas of atrophic, pseudohyperplastic, and foamy variants do not appear to be different from usual acinar adenocarcinoma and typically behave as conventional low-grade acinar Gleason score 6 prostate cancers in terms of patient outcome after radical prostatectomy. 21, 22, 23 Mucinous (colloid) adenocarcinoma used to be thought to confer a worse prognosis. However, recent reports indicate that mucinous adenocarcinoma treated by radical prostatectomy is not more aggressive than usual acinar adenocarcinoma and may even be less aggressive. 24 Prostate adenocarcinomas of signet ring–cell or lymphoepithelioma-like variants are rare and usually have very poor clinical outcomes. Histologic variants Foamy gland carcinoma Pseudohyperplastic carcinoma Adenocarcinoma with atrophic features Adenocarcinoma with glomeruloid features Large duct carcinoma Mucinous (colloid) carcinoma Small cell neuroendocrine carcinoma Sarcomatoid carcinoma (carcinosarcoma) Signet ring–cell carcinoma Squamous and adenosquamous carcinoma Adenoid cystic–type basaloid carcinoma Urothelial carcinoma Non–acinar carcinoma variants of prostatic carcinoma account for about 5 to 10% of primary prostate carcinomas. These histologic variants or types include, according to the WHO, sarcomatoid carcinoma, ductal adenocarcinoma, urothelial carcinoma, squamous and adenosquamous carcinoma, basal cell carcinoma, neuroendocrine tumors, including small cell carcinoma, and clear cell adenocarcinoma. Ductal adenocarcinoma is the most common histologic variant of prostatic carcinoma. The incidence of ductal adenocarcinoma including both pure ductal adenocarcinoma and mixed ductal–acinar adenocarcinoma, is about 3% of all prostatic carcinomas, with mixed ductal–acinar adenocarcinoma being more common than pure ductal adenocarcinoma. In radical prostatectomy specimens, the ductal adenocarcinoma is composed of confluent masses of papillary and/or cribriform adenocarcinoma. The ductal adenocarcinoma is almost always intimately admixed with acinar adenocarcinoma. Microscopically, prostatic duct adenocarcinoma is characterized by pseudostratified columnar epithelium, and therefore, it has also been termed endometrioid, endometrial, papillary, or papillary ductal adenocarcinoma. The outcome for men with prostatic ductal adenocarcinoma is, in most studies, worse than that for men with usual prostatic acinar adenocarcinoma, probably because of the tumors’ higher clinical stage or Gleason grade. Some patients with this variant respond to radical prostatectomy, hormonal therapy, and radiation therapy. 25 Recently, several PCa histologic types with distinct clinicopathologic features have been redefined. These include intraductal carcinoma and prostate cancer with neuroendocrine differentiation. Intraductal carcinoma of the prostate (IDC-P) represents spread of invasive carcinoma into preexisting benign ducts and acini and is strongly associated with high-grade (Gleason grades 4 or 5), large-volume, invasive prostate cancers. 26 The glands of intraductal carcinoma of the prostate glands are larger than normal peripheral zone glands and exhibit markedly irregular and branching contours. In addition to the presence of malignant epithelial cells filling large acini and prostatic ducts with preservation of basal cells, the diagnosis of IDC-P requires the presence of a solid or dense cribriform pattern ( ▶ Fig. 2.5 a; ▶ Fig. 2.5 b). If these features are not present, a diagnosis of IDC-P can be made if there is nonfocal comedonecrosis involving > 2 glands 1, or marked nuclear atypia 2, whereby the nuclei are at least 6 times larger than adjacent benign nuclei. 26, 27 Fig. 2.5 (a) Intraductal carcinoma of the prostate displays a cribriform proliferation of malignant cells that distend the lumen of the large prostatic duct (solid circle) with preservation of benign basal cells (dashed oval). (b) Immunostains highlight the malignant cells (AMACR, red) and the preserved basal cells (high molecular weight keratin and P63, brown). Studies have established that IDC-P represents an aggressive form of PCa and is an adverse pathologic parameter in both radical prostatectomy and needle biopsy specimens. The presence of IDC-P is associated with other adverse pathologic features in radical prostatectomy specimens, including higher Gleason scores, larger tumor volumes, and greater probability of extraprostatic extension, seminal vesicle invasion, and pelvic lymph node metastasis. It is also associated with decreased biochemical progression–free survival and with postsurgical biochemical recurrence. Epstein et al reported cases of IDC-P in prostate biopsies without invasive carcinoma. 28 They found that the presence of IDC-P, even in the absence of documented invasive carcinoma, was associated with an aggressive clinical course and adverse pathologic findings in subsequent radical prostatectomy specimens. Based on their studies of needle biopsy with IDC-P and previous studies in the literature that demonstrated consistent association of IDC-P at radical prostatectomy with multiple adverse prognostic factors, definitive therapy was recommended in men with IDC-P on needle biopsy, even in the absence of pathologically documented invasive PCa. Neuroendocrine (NE) differentiation can occur de novo with or without concurrent PCa, or as a transformed phenotype emerging from prior treatment for prostate cancer. Neuroendocrine phenotype generally confers a more aggressive clinical behavior and less favorable prognosis than that of conventional PCa. To standardize the diagnosis and facilitate further study, a morphological classification of NE differentiation in PCa was proposed recently 29 and consists of 6 categories: (1) usual prostate adenocarcinoma with NE differentiation, (2) adenocarcinoma with Paneth-cell–like NE differentiation, (3) carcinoid tumor, (4) small cell carcinoma, (5) large cell NE carcinoma, and (6) mixed NE carcinoma–acinar adenocarcinoma. Usual PCa with NE differentiation refers to typical acinar or ductal PCa in which NE differentiation is demonstrated only by immunohistochemical positivity (synaptophysin, chromogranin A, and CD 56). The clinical significance of NE differentiation in these tumors is uncertain and most of the studies have shown no effect on outcomes. Prostate cancer with Paneth-cell–like differentiation is typical PCa containing changes resembling Paneth-cells (i.e., prominent eosinophilic cytoplasmic granules on light microcopy and neurosecretory granules on electron microscopy). The clinical significance of PCa with Paneth-cell–like differentiation is not completely understood, although studies have shown that seemingly poorly differentiated PCa with Paneth-cell–like differentiation has a favorable prognosis. Prostate carcinoid tumor is a well-differentiated NE tumor with the classical morphology of carcinoid tumor arising in the prostatic parenchyma. It expresses NE markers, but not PSA. It is exceedingly rare, and strict diagnostic criteria should be used. Small cell carcinoma is an aggressive NE tumor recognized by its typical morphology and immunoprofiles similar to small cell carcinoma of the lung. Large cell NE carcinoma is a high-grade NE tumor with both morphological NE features (large nests of tumor cells with peripheral palisading, non–small cell nuclear features) and extensive NE marker expression. The majority of cases represent progression from a prior typical PCa following long-standing androgen ablation. Mixed NE carcinoma and acinar PCa comprise distinct components of NE (small cell or large cell) carcinoma and typical acinar PCa with abrupt transition. Most, if not all, cases of mixed small cell carcinoma and PCa represent NE transformation after androgen-deprivation therapy, and are hormone resistant with a poor prognosis. The Gleason grading system, developed by Dr. Donald Gleason in 1967, remains the cornerstone for the management of prostate cancer. The system is relatively simple and reasonably reproducible. It may be considered the key parameter for planning treatment, as well as the most important prognostic factor in predicting pathologic findings at radical prostatectomy, biochemical failure (rise in PSA after treatment), local and distant metastasis after therapy, and PCa specific mortality. The system assigns histologic patterns 1 through 5, with 1 being the most differentiated and 5 the least differentiated. The Gleason score is defined as the sum of the most and second-most common Gleason patterns and ranges from 2 to 10. 30 It has undergone continuous modification in response to changes in the clinical practice of diagnosis and treatment of prostate cancer since its inception. 31, 32 The most significant changes were introduced in 2005 at the auspices of the International Society of Urological Pathology (ISUP), although further modifications also ensued. 31 The resulting contemporary grading system is referred to as the 2005 ISUP modified Gleason grading system ( ▶ Fig. 2.6). However, it is important to stress that the changes put forth by ISUP in 2005 simply codified what had already been used in practice by many pathologists. Fig. 2.6 The 2005 International Society of Urological Pathology (ISUP) modified classification of Gleason grading, showing the characteristic architecture of Gleason patterns 1 through 5. (Used with permission from The Trustees of Indiana University; illustration by Thomas Weinzerl.)
2.1.2 Anatomy and Histology of Prostate Cancer
2.1.3 Multifocality and Dominant Nodule in Radical Prostatectomy
2.1.4 Pathologically Insignificant and Significant Prostate Cancer
2.2 Prostate Cancer
2.2.1 Prostate Cancer and Histologic Variants
Intraductal Carcinoma of the Prostate
Prostate Cancer with Neuroendocrine Differentiation
2.2.2 Clinically Significant Cancer
Gleason Grading System
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree