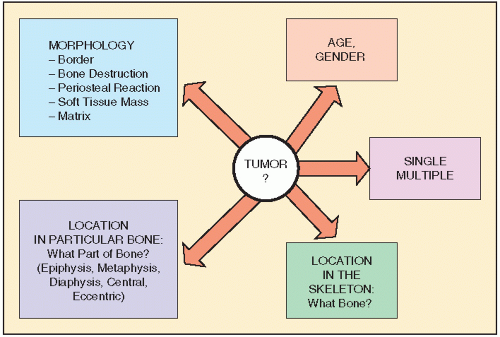
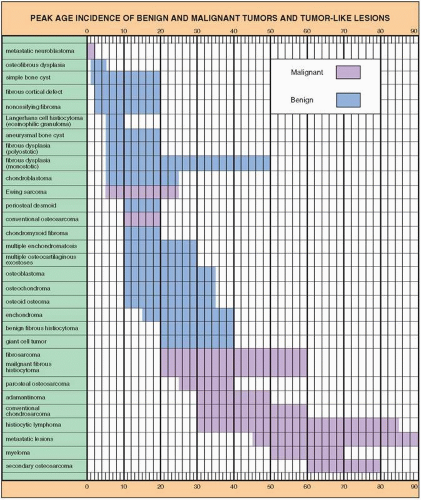
Computed Tomography
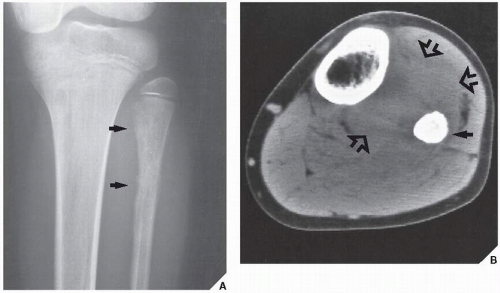
Although CT by itself is rarely helpful in making a specific diagnosis, it can provide a precise evaluation of the extent of a bone lesion and may demonstrate breakthrough of the cortex and involvement of surrounding soft tissues (
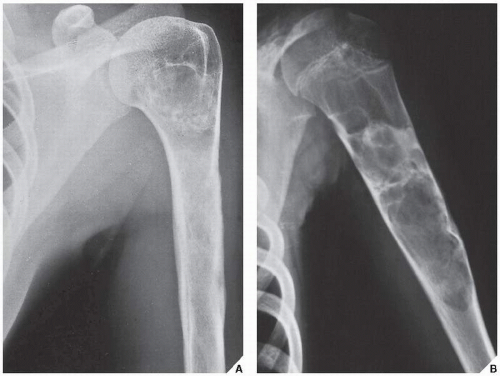
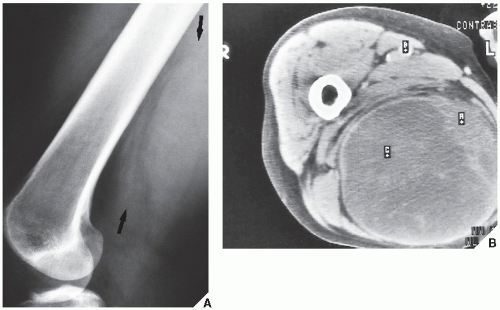
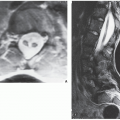
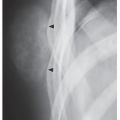
Fig. 16.7). CT is moreover very helpful in delineating a bone tumor having a complex anatomic structure. The scapula (
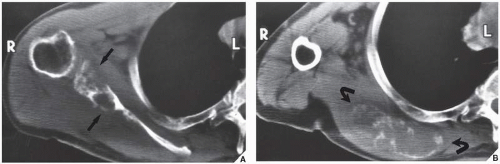
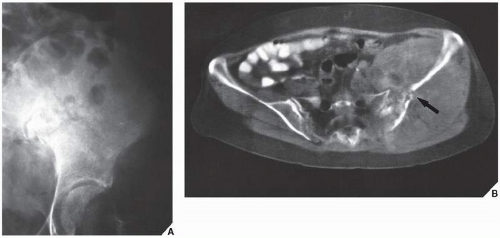
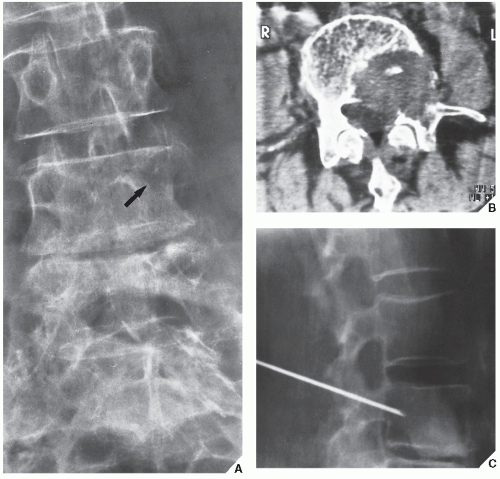
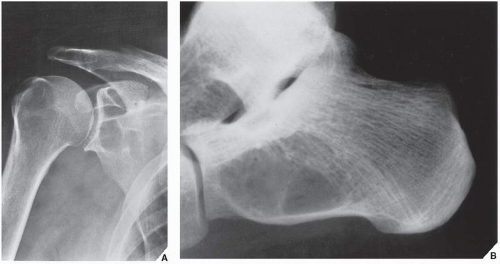
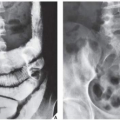
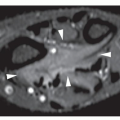
Fig. 16.8), pelvis (
Fig. 16.9), and sacrum, for example, may be difficult to image fully with conventional radiographic techniques. At times, three-dimensional computed tomography (3D CT)-reconstructed images are used to better and more comprehensively demonstrate the tumors. This technique can be useful, for example, in depicting surface lesions of bone, such as osteochondroma (
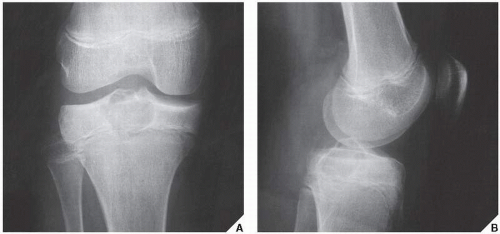
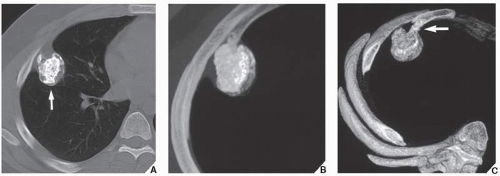
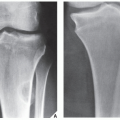
Fig. 16.10), parosteal osteosarcoma, or juxtacortical chondrosarcoma. CT examination is crucial in determining the extent and spread of a tumor in the bone if limb salvage is contemplated, so that a safe margin of resection can be planned (
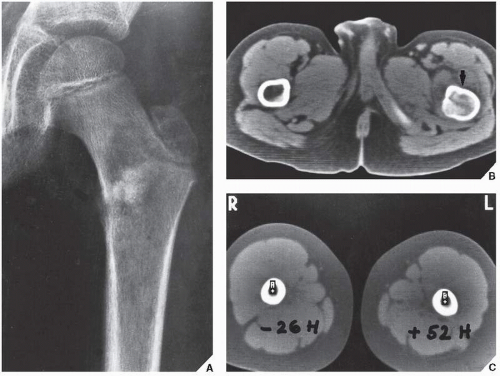
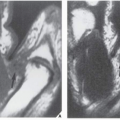
Fig. 16.11). It can effectively demonstrate the intraosseous extension of a tumor and its extraosseous involvement of soft tissues such as muscles and neurovascular bundles. CT is also useful for monitoring the results of treatment, evaluating for recurrence of a resected tumor, and demonstrating the effect of nonsurgical treatment such as radiation therapy or chemotherapy (
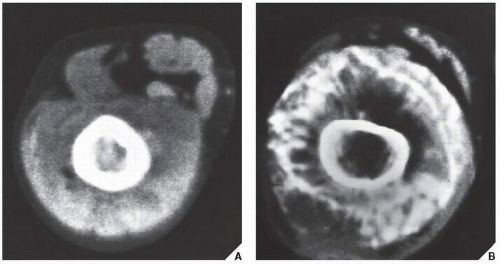
Fig. 16.12). It is also helpful in evaluating soft-tissue tumors (
Fig. 16.13), which on standard radiographs are indistinguishable from one another (with the exception of lipomas, which usually demonstrate low-density features), blending imperceptibly into the surrounding normal tissue.
Contrast enhancement of CT images aids in the identification of major neurovascular structures and well-vascularized lesions. Evaluating the relationship between the tumor and the surrounding soft tissues
and neurovascular structures is particularly important for planning limb-salvage surgery.
Arteriography
Arteriography is used mainly to map out bone lesions and to assess the extent of disease. It is also used to demonstrate the vascular supply of a tumor and to locate vessels suitable for preoperative intra-arterial chemotherapy, as well as to demonstrate the area suitable for open biopsy because the most vascular area of a tumor contains the most aggressive
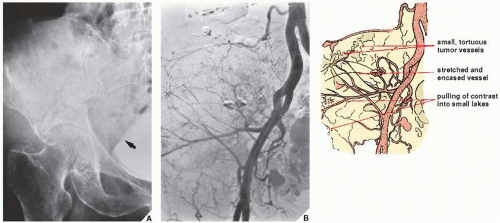
component. Occasionally, arteriography can be used to demonstrate abnormal tumor vessels, corroborating findings with conventional radiography (
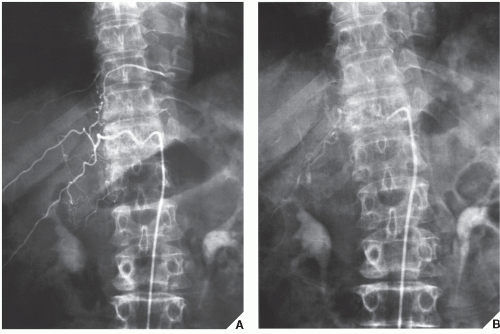
Fig. 16.16). Arteriography is often useful in planning for limb-salvage procedures because it demonstrates the regional vascular anatomy and thus permits a plan to be drawn up for the resection procedure. It is also sometimes used to outline the major vessels before resection of a benign tumor (
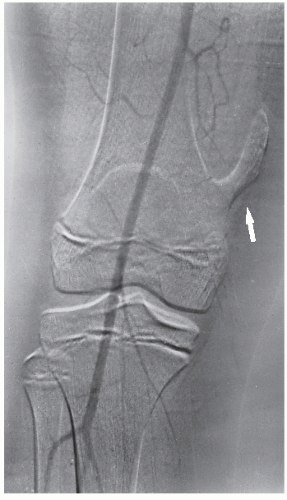
Fig. 16.17), and it can be combined with an interventional procedure, such as embolization of hypervascular tumors, before further treatment (
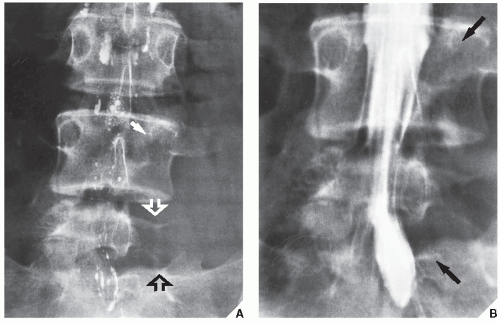
Fig. 16.18). In selected cases, arteriography may help make a differential diagnosis, such as of osteoid osteoma versus a bone abscess.
Magnetic Resonance Imaging
MRI is indispensable in evaluating bone and soft-tissue tumors. Particularly with soft-tissue masses, MRI offers distinct advantages over CT. There is improved visualization of tissue planes surrounding the lesion, for example, and neurovascular involvement can be evaluated without the use of intravenous contrast.
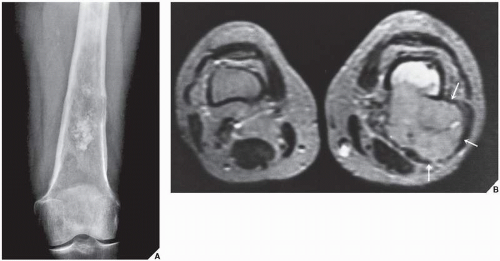
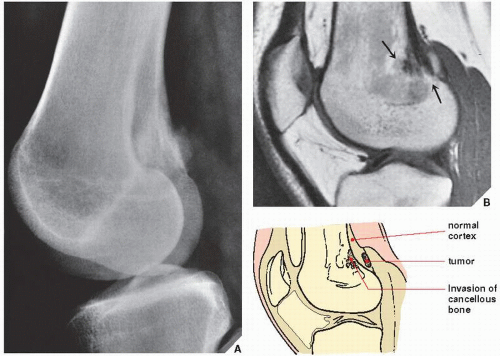
In the evaluation of intraosseous and extraosseous extensions of a tumor, MRI is crucial because it can determine with high accuracy the presence or absence of soft-tissue invasion by a tumor (
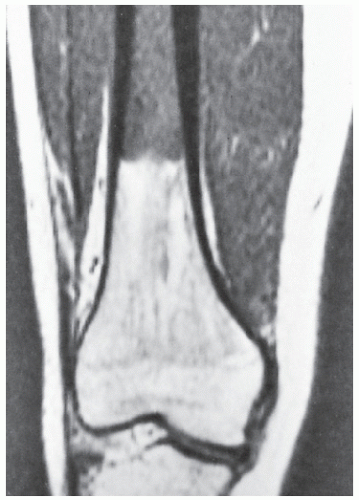
Fig. 16.20). MRI has often proved to be superior to CT in delineating the extraosseous and intramedullary extent of the tumor and its relationship to surrounding structures (
Fig. 16.21). By showing sharper demarcation between normal and abnormal tissue than CT, MRI—particularly in evaluation of the extremities—reliably identifies the spatial boundaries of tumor masses (
Fig. 16.22), the encasement and displacement of major neurovascular bundles, and the extent of joint involvement. Spin-echo
T1-weighted images enhance tumor contrast with bone, bone marrow, and fatty tissue, whereas spin-echo T2-weighted images enhance tumor contrast with muscle and accentuate peritumoral edema. Axial and coronal images have been used in determining the extent of soft-tissue invasion in relation to important vascular structures. However, in comparison with CT, MR images do not clearly demonstrate calcification in the tumor matrix; in fact, large amounts of calcification or ossification may be almost undetectable. Moreover, MRI has been shown to be less satisfactory than CT in the demonstration of cortical destruction. It is important to realize that both MRI and CT have advantages and disadvantages, and circumstances exist in which either can be the preferential or complementary study. But it is even more important that the surgeon
tell the radiologist who is performing and interpreting the study what information is needed.
Several investigators have stressed the superior contrast enhancement of MR images using intravenous injection of gadopentetate dimeglumine (gadolinium diethylenetriamine-penta-acetic acid, [Gd-DTPA]). Enhancement was found to give better delineation of the tumor’s richly vascularized parts and of the compressed tissue immediately surrounding the tumor. It was also found to assist in the differentiation of intraarticular tumor extension from joint effusion, and, as Erlemann pointed out, improved the differentiation of necrotic tissue from viable areas in various malignant tumors.
According to the recent investigations, MRI may have an additional application in evaluating both the tumor’s response to radiation and chemotherapy and any local recurrence. On gadolinium-enhanced T1-weighted images, signal intensity remains low in avascular, necrotic areas of tumor while it increases in viable tissue. Although static MRI was of little value for the assessment of response to the treatment, dynamic MRI using Gd-DTPA as a contrast enhancement, according to Erlemann, had the highest degree of accuracy (85.7%) and was superior to scintigraphy, particularly in patients who were receiving intra-arterial chemotherapy. In general, drug-sensitive tumors display slower uptake of Gd-DTPA after preoperative chemotherapy than do nonresponsive lesions. As Vaupel contended, the rapid uptake of Gd-DTPA by malignant tissues may be due to increased vascularity and more rapid perfusion of the contrast material through an expanded interstitial space. The latest observation by Dewhirst and Kautcher suggests that MR spectroscopy may also be useful in the evaluation of patients undergoing chemotherapy.
It must be stressed, however, that most of the time MRI is not suitable for establishing the precise nature of a bone tumor. In particular, too much faith has been placed in MRI as a method of distinguishing benign lesions from malignant ones. An overlap between the classic characteristics of benign and malignant tumors is often observed. Moreover, some malignant bone tumors can appear misleadingly benign on MR images and, conversely, some benign lesions may exhibit a misleadingly malignant appearance. Attempts to formulate precise criteria for correlating MRI findings with histologic diagnosis have been largely unsuccessful. Tissue characterization on the basis of MRI signal intensities is still unreliable. Because of the wide spectrum of bone tumor composition and their differing histologic patterns, as well as in tumors of similar histologic diagnosis, signal intensities of histologically different tumors may overlap or there may be variability of signal intensity in histologically similar tumors.
Trials using combined hydrogen-1 MRI and P-31 MR spectroscopy also failed to distinguish most benign lesions from malignant tumors. Despite the use of various criteria, the application of MRI to tissue diagnosis has rarely brought satisfactory results. This is because, in general, the small number of protons in calcified structures renders MRI less effective in diagnosing bone lesions, and hence valuable evidence concerning the production of the tumor matrix can be missed. Moreover, as several investigations have shown, MRI is an imaging modality of low specificity. T1 and T2 measurements are generally of limited value for histologic characterization of musculoskeletal tumors. Quantitative determination of relaxation times has not proved to be clinically valuable in identifying various tumor types, although, as noted by Sundaram, it has proved to be an important technique in the staging of osteosarcoma and chondrosarcoma. T2-weighted images in particular are a crucial factor in delineating extraosseous tumor extension and peritumoral edema, as well as in assessing the involvement of major neurovascular bundles. Necrotic areas change from a low-intensity signal in the T1-weighted image to a very bright, intense signal in the T2-weighted image and can be differentiated from viable, solid tumor tissue. Although MRI cannot predict the histology of bone tumors, as Sundaram pointed out, it is a useful tool for distinguishing round cell tumors and metastases from stress fractures or medullary infarcts in symptomatic patients with normal radiographs, and, according to Baker, it can occasionally differentiate benign from pathologic fracture.
Skeletal Scintigraphy
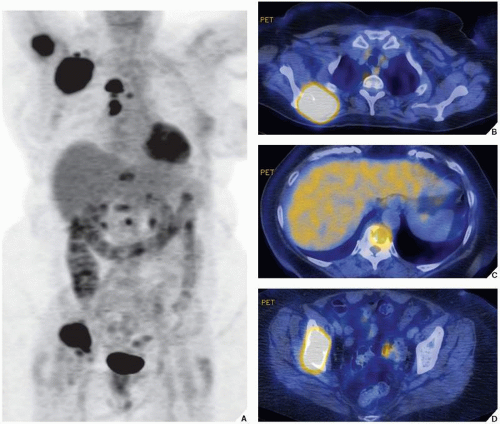
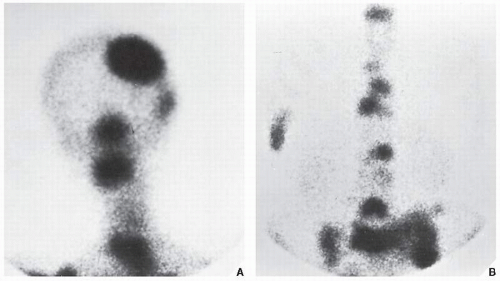
The radionuclide bone scan is an indicator of mineral turnover, and because there is usually enhanced deposition of bone-seeking radiopharmaceuticals in areas of bone undergoing change and repair, a bone scan is useful in localizing tumors and tumor-like lesions in the skeleton, particularly in such conditions as fibrous dysplasia, Langerhans cell histiocytosis, or metastatic cancer, in which more than one lesion is encountered (
Fig. 16.23). It also plays an important role in localizing small lesions such as osteoid osteomas, which may not always be seen on conventional radiographs (see
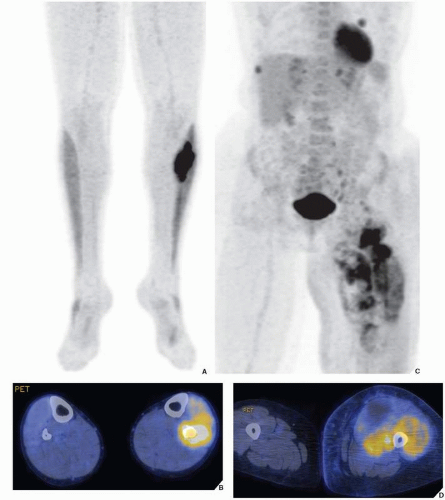
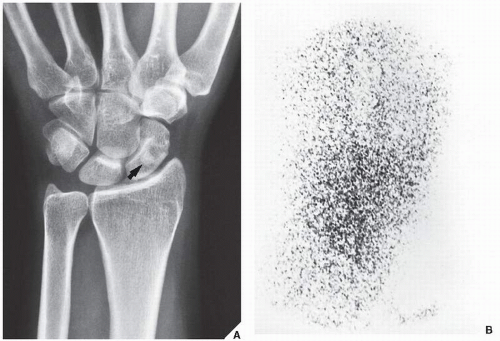
Fig. 17.11B). Although in most instances, a radionuclide bone scan cannot distinguish benign lesions from malignant tumors, because increased blood flow with increased isotope deposition and increased osteoblastic activity takes place in benign and malignant conditions, it is still occasionally capable of making such differentiation in benign lesions that do not absorb the radioactive isotope (
Fig. 16.24). The radionuclide bone scan is sometimes also useful for differentiating multiple myeloma, which usually shows no significant uptake of the tracer, from metastatic cancer, which usually does.
Aside from routine radionuclide scans performed using 99mTc-labeled phosphate compounds, occasionally 67Ga is used for the detection and staging of bone and soft-tissue neoplasms. Gallium is handled by the body much like iron in that the protein transferrin carries it in the plasma, and it also competes for extravascular iron-binding proteins such as lactoferrin. The administered dose for adults ranges from 3 mCi (111 MBq) to 10 mCi (370 MBq) per study. The exact mechanism of tumor uptake of gallium remains unsettled, and its uptake varies with tumor type. In particular, Hodgkin lymphomas and histiocytic lymphomas are prone to significant gallium uptake.