Fig. 1
Origin of registered neuroendocrine tumors
Patients were heavily pretreated. In 218 patients, number and kind of previous therapies were documented and included in the registry. Of these 218 patients, 44 (20%) experienced no previous therapy, while the rest had undergone between one and eight previous therapies. Most patients were treated by 1–2 therapies (122 patients, 55%), 20% (45 patients) by 3–4 therapies, whereas 5–8 therapies had seldom been used and performed in only 7 patients (3%). Mostly surgery (125/218 patients, 57%) or medical therapy (114/218 patients, 52%) was performed, while local ablative therapies were used as pretreatment in 20/218 patients (9%) and radiotherapy (external beam) in 14/218 patients (6%).
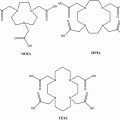
Between 1 and 8 cycles of peptide receptor radionuclide therapy were administered (median 3, mean 3.07, standard deviation 1.5) using mainly lutetium-177 (53%, 160/297) and yttrium-90 (46%, 137/297). Two patients were treated with gallium-67 radionuclide. As somatostatin analogs and chelators, mostly [DOTA0, Tyr3] octreotate (DOTATATE) and [DOTA0, Tyr3] octreotide (DOTATOC) were applied, in 91% and 8% of therapy cycles, respectively, whereas in 1% of cycles other ligands and chelators were used.
For the first cycle of peptide receptor radionuclide therapy, median activity was 3.75 GBq in 134 patients treated with yttrium-90 (mean 3.79 GBq, range 1.5–8.14 GBq) and 7.4 GBq in the 160 patients treated with lutetium-177 (mean 6.93 GBq, range 0.629–7.4 GBq). Cumulative dose was in median 7.35 GBq in 66 patients treated solely with yttrium-90 (mean 7.9 GBq, range 2–19 GBq) and 22 GBq for the 133 patients treated exclusively with lutetium-177 (mean 21.2 GBq, range 2.6–51.9 GBq).
Peptide receptor radionuclide therapy was not continued in 190 of 297 patients. Of these patients, response status was known in 151 patients. Any kind of remission was noted in 20% of patients (5% complete remission, 14% partial remission, 0.5% minor remission), stable disease in 48%, and progressive disease in 10% of patients. Among all 297 patients, 19 deaths were noted. Mean overall survival was estimated at 213 months with follow-up between 1 and 230 months after initial diagnosis, and 87 months with follow-up between 1 and 92 months after start of peptide receptor radionuclide therapy. Median overall survival was not yet reached (Fig. 2a, b).
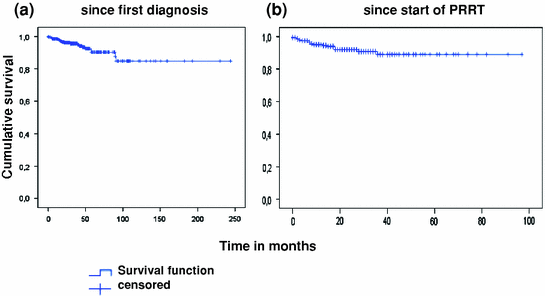

Fig. 2
Overall survival of all patients after initial diagnosis (a) and after start of peptide receptor radionuclide therapy (b)
Subgroup analysis was performed according to tumor grade, number of previous therapies, and grading of the neuroendocrine tumors by Ki67-based proliferation rate. Of 293 patients, 38 had proliferation index <2% (G1; 13%), 97 had proliferation index 2–20% (G2; 33%), and 16 had proliferation index >20% (G3; 5%). In almost half of cases, proliferation rates were not documented (142/293; 48%). Subgroup analysis demonstrated that best results were obtained in grade 1 and 2 neuroendocrine tumors with proliferation rate below 20%. Interestingly, results were similar in the group without known proliferation rate and the group with proliferation rate higher than 20% (G3; Fig. 3a, b).
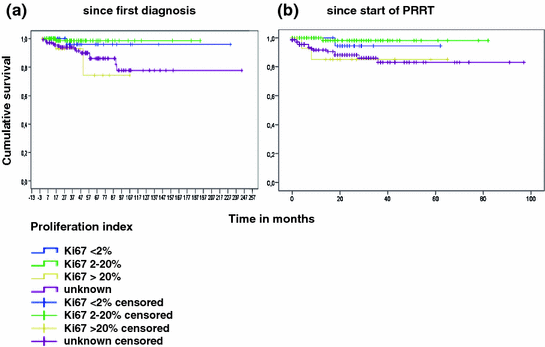

Fig. 3
Overall survival according to tumor grading determined by Ki67 index after initial diagnosis (a) and after start of peptide receptor radionuclide therapy (b)
Overall survival was not dependent upon number of previous therapies. Most patients with documented previous therapies had had two or three therapies (90/293; 30%). No previous therapy was noted in 43/293 patients (14%), 66 (22%) had had one previous therapy, and 17/293 (5%) more than three previous therapies. No information could be assessed for 77/293 (26%). Although most patients were pretreated, no difference in survival could be documented depending on the number of pretreatments (Fig. 4a, b). Best results were obtained in patients with documented previous therapies, whereas unknown pretreatments appeared to be a risk factor in survival analysis.
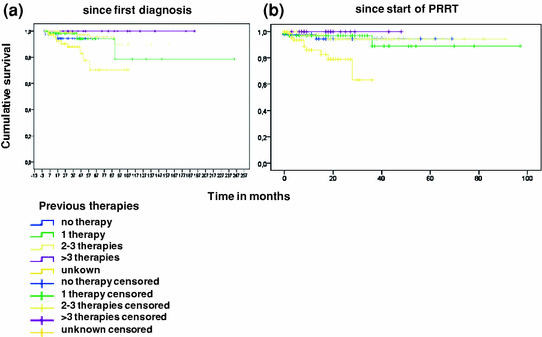

Fig. 4
Overall survival according to previous therapies after initial diagnosis (a) and after start of peptide receptor radionuclide therapy (b)
4 Discussion
Our results indicate that peptide receptor radionuclide therapy is effective and safe for treatment of well- and moderately differentiated neuroendocrine tumors. Although, the registry so far does not contain long-term follow-up, mean follow-up of registered patients was 19.6 months, resulting in long mean survival times of more than 200 months after first diagnosis and more than 80 months after start of peptide receptor radionuclide therapy. Scheduled follow-ups at 24-month intervals will complete these promising data in the next years. Survival curves presented in this preliminary analysis indicate that prognosis of neuroendocrine tumor patients treated by peptide receptor radionuclide therapy is excellent. Since patients treated by peptide receptor radionuclide therapy undergo a selection process prior to therapy, these data may represent positive selection according to slow tumor progression as well as effects of peptide receptor radionuclide therapy on tumor progression. To date, registered data on side-effects of peptide receptor radionuclide therapy do not indicate serious side-effects on kidney and bone marrow function, data which are especially sensitive to close follow-up of treated patients in the coming years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree