(1)
Laboratory for Optical and Computational Instrumentation and Laboratory of Molecular Biology, University of Wisconsin, Madison, WI, USA
Abstract
The first practical laser scanning confocal microscopes were introduced to the biomedical community over 30 years ago. Their subsequent development continues to influence the introduction of new methods and applications of optical sectioning microscopy.
Key words
ConfocalLaser scanningOptical sectionFluorescenceLiving tissueThree-dimensional imaging1 Introduction
The three-dimensional nature of biological structures has presented a fundamental challenge to microscopists over the years. This is because images are often obscured by interfering signals from out-of-focus structures. A traditional approach was to immobilize the specimen by chemical fixation or freezing, and cutting thin sections of it. The sections were then examined sequentially to provide a three-dimensional reconstruction.
In recent years a number of optical sectioning techniques have become available which avoid the necessity to cut sections and thereby open up the possibility of visualizing three-dimensional structures in live specimens. Confocal microscopy is perhaps the most widely used of these techniques. It works by sequentially illuminating a restricted region of the specimen and excluding light that emanates from outside this region (Fig. 1).


Fig. 1
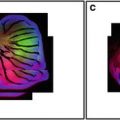
Confocal microscopy comes of age. Comparison of epifluorescence microscopy and laser scanning confocal imaging of a HeLa cell mitotic spindle labeled using indirect immunofluorescence with anti-tubulin (primary antibody) and a fluorescein labeled secondary antibody. The specimen was imaged using (a) conventional epifluorescence light microscopy and (b, c) using the laser scanning confocal microscope. Note the improved resolution of microtubules in the two confocal images (b) and (c) as compared with the conventional image (a). Image (b) was collected with the pinhole set to a wider aperture than image (c). (Image kindly provided by Brad Amos, University of Cambridge, UK). Scale bar = 5 μm
In a typical instrument, illumination is restricted to a defined structure within the specimen, such as a single spot, an array of spots, or an array of lines. A spatial filter (in the shape of the illuminated structure) is situated in the imaging path, which acts to block light that emanates from regions away from that which is illuminated. Images are built up by scanning the illuminating structure in tandem with the spatial filter so that the whole two-dimensional field is covered.
The basic idea for confocal microscopy was first put forward in a patent by Marvin Minsky in 1957 [1, 2], but it took the development of lasers, microfabrication techniques, and computer image acquisition systems before practical instruments became available to the biomedical community in the late 1980s and early 1990s [3–6].
2 The Laser Scanning Confocal Microscope
The rapid acceptance of confocal microscopy in the late 1980s by the biomedical research community was extraordinary for such a novel and relatively expensive technology. Indeed, the journal Science ran a cartoon of a little girl asking Santa Claus for a laser-scanning microscope for Christmas!
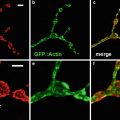
Perhaps the reason for this rapid acceptance was that standard specimen preparation techniques could be used. By far the most common mode of confocal microscopy used by the biomedical community is fluorescence. There are many reasons for this, such as the high signal-to-background ratio of fluorescence, the ability to use multiple labels and the increasing usage of genetically engineered fluorescent proteins [7].
In the early days of using the instruments we used to have conventional fluorescence set up on our confocal microscopes so that people could rapidly switch from conventional to confocal imaging (Fig. 2).


Fig. 2




The author, John White, photographed in the late 1980s seated at an early version of the MRC 500 laser scanning confocal microscope. In the top panel, John is looking into the eyepieces of the fluorescence microscope in order to find a region of interest for scanning. In the lower image the specimen is being scanned by the laser and the image produced by the confocal microscope is visible on the video monitor. The ability to first locate a region of interest by eye using a conventional wide field epifluorescence microscope, and to subsequently scan the same region in the confocal mode, remains a significant practical advantage of the laser scanning confocal microscope
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree