Gregory Nadolski • S. William Stavropoulos
Angiomyolipomas (AMLs) are a benign neoplasm of the kidney composed of varying mixtures of adipose, muscle, and vascular tissues. Although benign, the vasculature within these tumors develops abnormally and may spontaneously hemorrhage. The incidence of massive spontaneous hemorrhage increases with the degree of vascularity and size of the AML. Spontaneous massive hemorrhage can be managed with emergent nephrectomy. More recently, transarterial embolization has emerged as a preferred nephron-sparing option. To prevent the complication of hemorrhage, renal AMLs have been historically removed using nephrectomy or partial nephrectomy. More recently, prophylactic embolization of AMLs has become the treatment of choice to prevent AML hemorrhage once the tumor has grown to a sufficient size. In this chapter, the surveillance and treatment of AMLs will be discussed with a focus on indications, techniques, and outcomes of percutaneous transarterial embolization.
BACKGROUND
Histopathology and Epidemiology
Originally believed to be hamartomas with abnormal proliferation of tissues normally present in the kidney, AMLs are now classified as neoplasms of adipose, vascular, and smooth muscle tissue.1 These lesions arise from clonal proliferation of perivascular epithelioid cells, which first develop into small nodules of spindle cells within the capsule, cortex, or medulla of the kidney.1 As these neoplasms grow, they may develop into one of two basic forms of AML: typical and epithelioid.
First described in 1900 by Grawitz, typical AMLs consist of varying amounts of adipose, vascular, and smooth muscle tissue.2 The blood vessels within typical AMLs are structurally abnormal. They contain no internal elastic lamina, and the smooth muscle of their media is replaced by fibrous tissue. Thus, the vascular tissue of AMLs is tortuous, rigid, and prone to microaneurysm and macroaneurysm formation.3 The epithelioid variant has little if any adipose tissue and few blood vessels. Instead, this variant is composed almost entirely of epithelioid cells and behaves more aggressively than typical AMLs.2 Given the difficulty in differentiating epithelioid AMLs on imaging from other solid renal neoplasms such as renal cell carcinoma, epithelioid AMLs are almost exclusively managed surgically, and the diagnosis is often suspected clinically and confirmed postoperatively.4 Because epithelioid AMLs are rare and the imaging features and biologic behavior are different from typical AMLs, the subsequent discussion of AMLs will refer only to the typical variety.
Renal AMLs may occur sporadically or in association with tuberous sclerosis complex or lymphangioleiomyomatosis (LAM).2 The overall incidence of sporadic renal AMLs is now thought to be more common than once appreciated, with a frequency of about 13 per 10,000 adults (0.13%).1 Autopsy data from individuals without stigmata of TSC has demonstrated frequencies of 0.02% of males and 0.29% of females in the total population.5 Similarly, ultrasound screening of healthy individuals for incidental AMLs have demonstrated an incidence of 0.1% in males and 0.22% in females.6 Although some speculate the differences in incidence between sexes may be related to fewer males in most studies, others suggest the consistently observed higher frequency in females may be related to hormonal differences, which has been supported by the frequent presence of estrogen and progesterone receptors within AMLs.7
Tuberous sclerosis, also known as tuberous sclerosis complex (TSC), is a rare autosomal dominantly inherited multisystem disease characterized by tumors in the brain, kidneys, heart, lungs, and skin, which classically presents in childhood with seizures, developmental delay, behavioral problems, and skin abnormalities. These patients are prone to forming cysts, AMLs, and malignant neoplasms in the kidney. AMLs can be detected in children with TSC, and the number and size of these lesions continue to progress as the patient ages. Autopsy data on patients with TSC suggest the incidence of AMLs in this population may approach 67%.8
Clinical Symptoms and Indications for Intervention
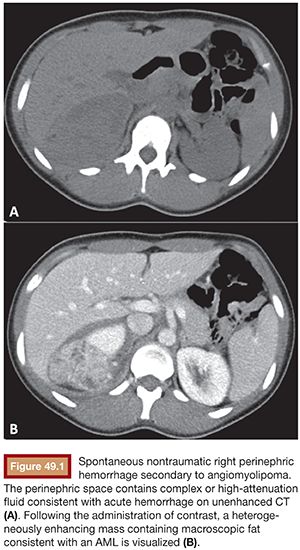
Although renal AMLs may be asymptomatic, they typically manifest clinically in one of two ways. First, patients may present with spontaneous, nontraumatic renal hemorrhage, which when confined to the subcapsular and perirenal space is referred to as Wunderlich syndrome (Fig. 49.1). Alternatively, and much less commonly, AMLs may slowly and progressively invade the normal renal parenchyma, resulting in renal failure.9,10 The latter manifestation is usually confined to patients with syndromes resulting in numerous renal AMLs, whereas hemorrhage may occur in either sporadic or syndromic AMLs. Although benign, renal AMLs rarely can invade the renal vein and inferior vena cava, placing the individual at risk for caval thrombosis and pulmonary embolism.4,11 This rare presentation of typical AMLs is most commonly associated with tumors measuring greater than 9.5 cm in diameter and found in women.
Hemorrhage within an AML results from rupture of microaneurysms and macroaneurysms within the tumor vasculature, which can subsequently bleed into the retroperitoneum. Other less common symptoms of AML include pain, flank mass, and hematuria.12 Overall, symptoms occur in 73% of patients with sporadic AMLs and 64% of patients with TSC-related AMLs, and hemorrhage is the presenting symptom in 14% and 44% of patients with sporadic and TSC renal AMLs, respectively.12
The risk of hemorrhage within an AML has been associated with both overall size of the AML as well as the size of the aneurysms within them. Most clinicians use overall size greater than 4 cm as an indication for prophylactic embolization, which has been supported by several studies. In a review of 253 AMLs, Oesterling et al.13 found 82% of AMLs with a diameter of 4 cm or larger were symptomatic, whereas only 23% of tumors smaller than 4 cm were symptomatic. Furthermore, of all symptomatic AMLs, 90% were at least 4 cm in diameter. Over half of AMLs 4 cm or larger bled, and one-third of the patients with acute hemorrhage presented in shock.13 Similarly, based on retrospective review of computed tomography (CT) of 29 kidneys with AMLs, 8 with and 21 without hemorrhage, tumor size larger than 4 cm and aneurysm size equal to 5 mm or larger were present in all hemorrhagic lesions.14 However, in this same study, aneurysm size of 5 mm or greater was more specific for tumor hemorrhage than overall size (86% vs. 38%), although both metrics had 100% specificity.14 Other series have shown no difference in the size of AMLs in patients who were treated after presenting with flank pain compared to those presenting with hemorrhage, further supporting the theory that aneurysm size is more important than overall AML size in determining risk of hemorrhage.15 Interestingly, size greater than 4 cm at presentation has been shown to correlate with a higher rate of subsequent growth (46% vs. 27%) and higher likelihood for eventual intervention (53.8% vs. 7%) compared to smaller AMLs, underscoring the value of the 4-cm threshold for intervening on asymptomatic AMLs.
Management and Treatment Options
Whereas all symptomatic AMLs are treated to alleviate symptoms, asymptomatic AMLs can be managed with surveillance until growth in size or development of aneurysmal vascularity necessitates intervention because these neoplasms have no malignant potential. Regardless of the method of intervention, optimum treatment requires preservation of renal function.15 Originally, surgical intervention, either total or partial nephrectomy, was employed to prevent hemorrhage. Subsequently, percutaneous embolization was developed to offer a minimally invasive nephron-sparing approach to management. Recently, oral medications targeting the mammalian target of rapamycin (mTOR) have shown promise in slowing or regressing the growth of AMLs. Following a discussion of imaging and imaging surveillance, medical and surgical options for treating AMLs will be discussed briefly, followed by detailed discussion of AML embolization technique and outcomes.
Diagnostic Imaging Techniques and Surveillance
When imaging a potential AML, the most important consideration is differentiating an AML from a malignant renal neoplasm. The distinction is particularly important for small masses because management of asymptomatic AMLs less than 4 cm involves imaging surveillance, whereas a malignancy would be managed with surgery or ablation. AMLs can be diagnosed using ultrasound, CT, or magnetic resonance imaging (MRI). In all three modalities, the diagnosis is primarily based on detection of macroscopic fat within a renal mass.
Classically on ultrasound, the fat-containing AMLs appear as a strongly hyperechoic mass with acoustic shadowing. However, lipid-poor AMLs are isoechoic and difficult to differentiate from adjacent parenchyma.16–18 Furthermore, there is significant overlap in the ultrasound appearance of AMLs with that of renal cell carcinoma, particularly for lesions less than 3 cm, which limits its use in the surveillance of presumed AMLs.18,19 Color and power Doppler have been used to improve the diagnostic accuracy of ultrasound for the detection of AMLs, but the diagnostic accuracy only approaches 83% for lesions measuring under 3 cm.20 Although ultrasound can detect hemorrhage in the perinephric space with great sensitivity, it may not be able to definitively diagnose the presence of an AML in the acute setting, which would require further evaluation with CT or MRI.
Because there is significant overlap of the ultrasound features of AML and renal cell carcinoma, CT is often the preferred method of diagnosing AMLs. The diagnosis of AML by CT depends on visualizing macroscopic fat characterized by a negative Hounsfield unit (Fig. 49.1); however, other renal masses can contain fat, including renal cell carcinoma.16 Classically, AMLs appear as predominantly fatty masses similar in attenuation to the subcutaneous or retroperitoneal fat with various amounts of soft tissue scattered within it. However, the exact appearance of an AML depends on the exact proportion of the adipose, vascular, and smooth muscle tissue.13,16,21 If large areas of macroscopic fat cannot readily be visualized, use of thinner sections or pixel mapping can improve detection of fat within the mass.22,23 Although renal cell carcinoma has been reported to rarely contain fat, this is believed to be either secondary to tumor necrosis or trapping of renal sinus fat.24,25 Differentiating lipid-poor AMLs from renal cell carcinoma can be more challenging, but multiphase contrast-enhanced CT can be used to differentiate the two. The best predictor of a lipid-poor AML on CT is homogeneous prolonged enhancement, which has a positive predictive value of 91%.18 Last, CT is particularly useful in the setting of acutely symptomatic AMLs as it can accurately detect perinephric hemorrhage, possibly identify aneurysms within the mass, and distinguish AMLs from other sources of flank pain such as urolithiasis and pyelonephritis.
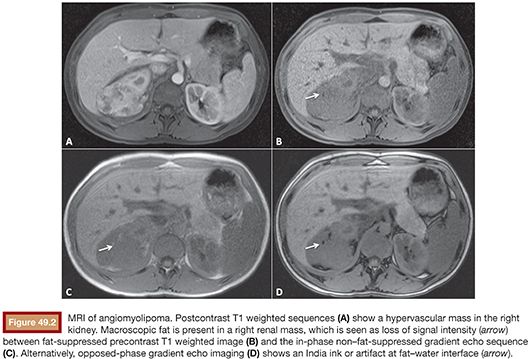
MRI is particularly well suited at detecting both macroscopic and microscopic fat with high accuracy, making it an ideal imaging modality for diagnosing and following AMLs. Classically, the presence of macroscopic fat in the mass can be imaged by assessing differences in signal intensity between fat-suppressed and non–fat-suppressed sequences. Alternatively, opposed-phase imaging, which typically is used to detect intracellular lipid in adrenal adenomas, can be used to characterize AMLs and differentiate them from renal cell carcinoma (Fig. 49.2). In particular, when using opposed-phase imaging, an India ink or etching artifact occurs because the presence of fat and water protons within the same voxel results in signal loss at fat–water interfaces. In a retrospective study, Israel et al.26 found 100% of the 23 AMLs studied demonstrated an India ink artifact between the AML and the adjacent normal renal parenchyma, allowing correct diagnosis in all cases while only mischaracterizing a single small renal cell carcinoma. Of note, the clear cell subtype of renal cell carcinoma is known to lose signal on opposed-phase imaging because of the presence of intracellular lipid; however, these lesions do not lose signal on fat-suppressed sequences. In their series, Israel et al.26 examined two clear cell renal cell carcinomas, neither of which demonstrated India ink artifact. Last, although lipid-poor AMLs do not contain a focus of macroscopic fat, these tumors do contain enough fat to be detected using chemical-shift imaging even if fat could not be detected on CT.27,28
Although all three modalities are acceptable for diagnosing AML, at our institution MRI is typically used to confirm the diagnosis, to perform active surveillance of incidental AMLs less than 4 cm, and to assess treatment response. MRI is particularly useful in patients with TSC. Following multiple interventions on multiple AMLs, these patients may have baseline chronic kidney disease, making contrast-enhanced CT less feasible. Although there is no consensus on how frequently surveillance imaging should be performed, asymptomatic AMLs less than 4 cm tend to remain stable in size and may be observed; thus, yearly surveillance is sufficient.29–31 This has been supported by additional investigations of a series of 91 patients with AML of which 73 were incidentally identified.32 In this series, 45 of the patients had small AMLs, which were observed with surveillance imaging. The mean AML growth rate was 0.09 cm per year.32 Three patients failed surveillance and underwent intervention, two with spontaneous hemorrhage and one with rapid growth of 0.7 cm per year. Periodic surveillance imaging of asymptomatic AMLs less than 4 cm appears to have low risk of failure given the relative stability of small AMLs. However, failure can occur, and given the incidence of hemorrhage in larger AMLs, asymptomatic lesions greater than 4 cm should undergo prophylactic elective treatment, which will be discussed subsequently.
Surgical and Medical Management of Angiomyolipomas
Surgical options to manage AMLs include total and partial nephrectomy. Partial nephrectomy has shown a similar success rate (near 100%) as total nephrectomy in treating AMLs without increased incidence of local recurrence during follow-up.12,32 Additionally, patients undergoing partial nephrectomy have been shown to have a significantly lower incidence of developing stage 3 or greater chronic kidney disease (defined as glomerular filtration rate <60 mL per minute) compared to those treated with total nephrectomy.33 However, despite the improvements in postprocedure renal function with partial nephrectomy, this procedure carries a total complication rate ranging between 5% and 23%.12,34 Additionally, the rate of conversion from partial to total nephrectomy may be higher when resecting AMLs compared to other lesions, although small series of laparoscopic partial nephrectomy showed no difference in complication rates or blood loss between AML and other renal neoplasms.34,35 Last, some studies suggest embolization of AMLs is preferable to partial nephrectomy in cases of acute hemorrhage and multifocal disease because of more rapid symptom control and better preservation of renal function, respectively.36 Alternatively, radiofrequency ablation (RFA), either laparoscopic or percutaneous image guided, has been employed as a less invasive nephron-sparing approach with outcomes similar to partial nephrectomy with possibly a reduction in complications.37
Although embolization or resection is the current standard of care for AMLs, which are symptomatic or greater than 4 cm, recent investigations of medical therapy using mTOR inhibitors have shown promise in causing regression of AML size. Additionally, investigators have begun employing multimodality therapy combining pretreatment with the mTOR inhibitor sirolimus followed by partial nephrectomy to further preserve posttreatment renal function.38 Once medical treatment is stopped, AMLs can continue to grow. In addition, long-term studies involving the effectiveness of medical therapy at decreasing the incidence of AML bleeding have not been done.
EMBOLIZATION MATERIALS AND TECHNIQUE
Renal Arteriography
Knowledge of the classic anatomy and variations of the renal vessels is important in the planning before AML embolization. Careful evaluation of preprocedural CT or MRI can be helpful in identifying variant anatomy.
Normally, the renal arteries arise from the abdominal aorta, below the origin of the superior mesenteric artery, typically at the level between the superior endplate of L1 and the inferior endplate of L2. Both renal arteries originate at the anterolateral aspect of the abdominal aorta, best profiled using a 10- to 30-degree left anterior oblique (LAO) projection. Near the renal hilum, each renal artery divides into an anterior and a posterior branch, which in turn divide into upper, middle, and lower pole segmental arteries. The segmental vessels bifurcate into lobar arteries, which penetrate the renal parenchyma and divide into the interlobar arteries. The interlobar arteries course between the renal pyramids and give rise to the arcuate arteries running parallel to the kidney surface. The arcuate arteries supply the interlobular arteries, which become the afferent arterioles entering the glomeruli.
Two types of variant renal arteries exist. Early division is a variant in which branching of the main renal arteries occurs more proximally than the hilum. The second type of variant is known as extrarenal arteries and can be further divided into two groups: accessory and aberrant arteries. Accessory arteries enter the kidney from the hilum along with the main renal artery, whereas aberrant arteries enter the kidney directly from the capsule, outside of the hilum. Accessory renal arteries are the most common and most clinically relevant vascular variant occurring in up to 30% of patient. Multiple renal arteries are unilateral in 30% of patients and bilateral in 10%. These accessory renal arteries usually arise from the aorta or iliac arteries anywhere from the level of T11 to the level of the L4 vertebra. In rare cases, these arteries can arise from other abdominopelvic or mesenteric arteries.39
Ethanol Plus Lipiodol Technique
Transarterial embolization of AML, performed under moderate sedation using fentanyl and midazolam, commonly employs the use of a mixture of ethiodized oil and ethanol mixed in a ratio of 3:7.40,41 Before the procedure, intravenous antibiotics, such as cefazolin 1 g, are administered before the procedure. After obtaining arterial access and placing a 5-Fr vascular sheath, an abdominal aortogram is obtained using a flush catheter positioned immediately inferior to the superior mesenteric artery to define the renal arterial anatomy using a 20 mL per second injection for a total volume of 20 mL. After aortography, selective renal catheterization is performed typically with a 5-Fr Cobra 2 catheter (Boston Scientific, Marlborough, Massachusetts) or a 5-Fr Sos reverse curve catheter (Angiodynamics, Latham, New York). If engaging or maintaining access to the renal origin is difficult secondary to atherosclerotic disease at the renal artery origin, tortuosity of the iliac vessels, or the obliquity of the renal artery origin, a long curved vascular sheath or alternative catheters such as the RC-2 (Angiodynamics, Latham, New York), Simmons, or Levin catheters (Cook Medical, Inc., Bloomington, Indiana) may be used to engage the origin or to better support access.
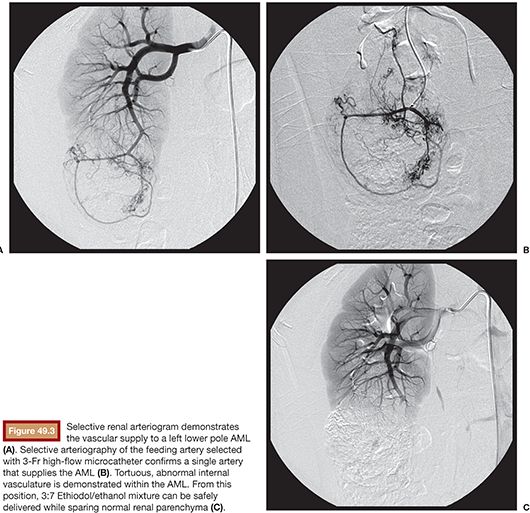
Selective renal arteriography is then performed using a 5 to 6 mL per second rate to determine the number of vessels feeding the AML (Fig. 49.3). Through the 5-Fr catheter, a high-flow 3-Fr microcatheter is advanced over a wire into a vessel supplying the tumor so that the microcatheter is distal to any branches supplying normal renal parenchyma. The guidewire is removed, and contrast material is injected to verify position using digital subtraction imaging. Additionally, the rate of contrast material injection is used to estimate the rate of Ethiodol/ethanol mixture injection, which can be used without causing reflux and nontarget embolization. At a separate sterile table designated for the embolic materials, the Ethiodol is mixed with the ethanol in a 3:7 ratio of Ethiodol to ethanol using polycarbonate syringes and metal three-way stopcocks. The feeding artery is then embolized until stasis is achieved. Postembolization control angiography is performed to determine the degree of embolization with the goal end points of occluding the arterial branches supplying the tumor and nonopacification of the tumor itself (Fig. 49.4). If residual tumor vascularity is visualized, additional Ethiodol/ethanol can be injected in the manner described earlier until satisfactory stasis is achieved. Additional branches supplying the mass can be catheterized selectively and the process repeated until the entire tumor is embolized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree