Fig. 9.1
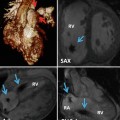
RV–PA cine views. Still frame from sagittal right ventricle outflow tract (RVOT) cine in diastole (ai) and systole (aii) is shown. There is mild residual subpulmonary valve RVOT obstruction with maximum velocity 2.1 m/s. The cross-cut RVOT cine view (b) is from a plane perpendicular to the pulmonary valve (PV) and RVOT on the sagittal RVOT cine (white bar) and is shown in diastole (bi) and systole (bii). Note also there is mild kinking of the distal conduit used in the most recent surgery associated with a peak velocity 1.8 m/s
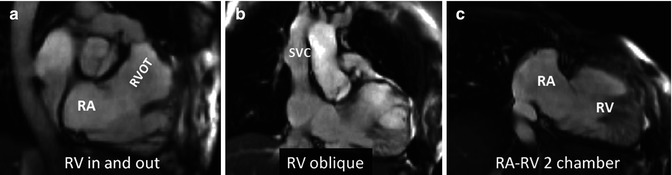
Fig. 9.2
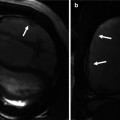
Additional long-axis views. Examples of right ventricle (RV) in and out (a), RV oblique (b) and right atrial–right ventricular (RA–RV) long-axis (c) cine orientations are shown. (a) and (b) are useful for detecting ventricular septal defect patch leaks, right ventricle outflow tract (RVOT) regional wall motion abnormality and a dilated RV less apparent in four-chamber orientation and for subsequent piloting of late enhancement CMR. These views may show the akinetic area of the RVOT and may be useful views in addition to a four-chamber and basal short axis for assessment of tricuspid regurgitation. SVC superior vena cava
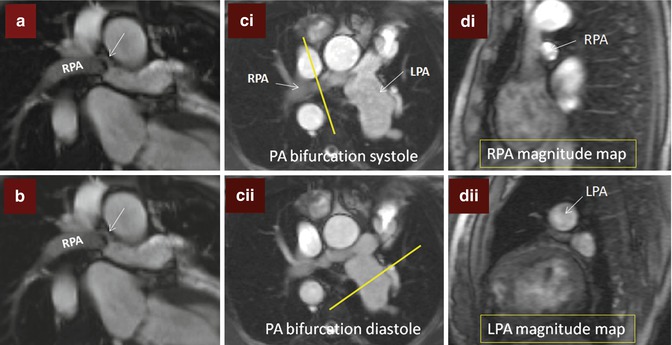
Fig. 9.3
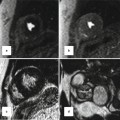
Branch pulmonary artery (PA) assessment. (a) Shows discrete mid right pulmonary artery (RPA) stenosis in a TOF patient with previous history of Waterston shunt. The shear edges of the mid RPA jet (arrow) can be seen. The RPA measured 9 × 5 mm at the level of stenosis where peak velocity was 2 m/s. (b) is a corresponding in-plane phase velocity map with right–left orientation useful to help determine the area of peak velocity and for subsequent peak velocity throughplane mapping location. (c) Images are of the PA bifurcation in systole (ci) and diastole (cii) showing relative lack of pulsatility of the RPA versus the left pulmonary artery (LPA) which is also dilated. The yellow bar in (ci) and (cii) shows the planes used to locate the throughplane RPA velocity map (magnitude image di) and LPA velocity map (magnitude image dii), respectively. Regions of interest drawn throughout the cardiac cycle on the corresponding phase encoded velocity maps can be used to determine the blood flow through the RPA and LPA and to subsequently calculate differential lung perfusion. In this case percentage RPA:LPA flow was reversed at 40:60. The features are of moderate RPA stenosis
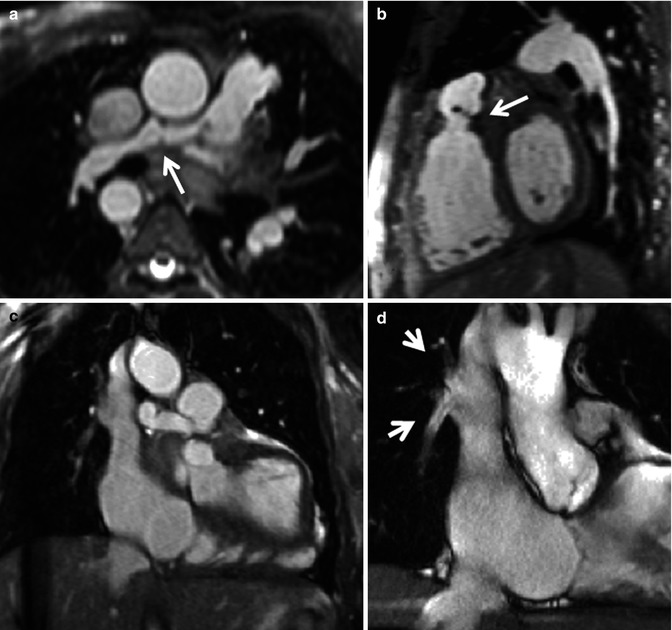
Fig. 9.4
Utility of 3D assessment. Still images from 3D balanced steady-state free precession (bSSFP) acquisition in diastole in transaxial (a), sagittal (b) and coronal (c) orientations. (a) Shows the distorted small right pulmonary artery (RPA) (arrow). (b) Shows a fixed pulmonary valve leaflet (arrow). (c) Shows anomalous right pulmonary venous drainage to the superior vena cava and a small field of view cine (d) was subsequently piloted to show the right upper lobe pulmonary vein and right middle lobe pulmonary vein returning to the superior vena cava (arrows in d). This was associated with a Qp:Qs of 1.3:1. The 3D data set was very useful to delineate the two small anomalous right-sided pulmonary venous vessels
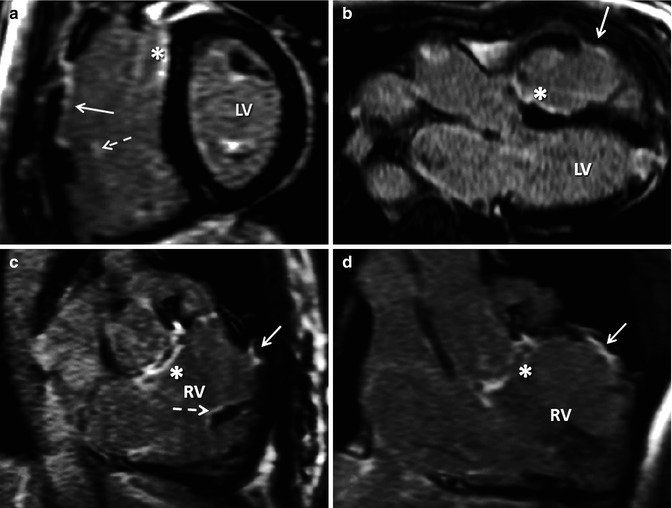
Fig. 9.5
Need for long-axis right ventricle (RV) views for late contrast enhancement (LGE) in TOF. A mid short-axis view is shown late after administration of intravenous gadolinium (a) demonstrating right ventricle outflow tract (RVOT) LGE with endocardial extension in the anterior RV free wall. There is also ventricular septal defect (VSD) site scar (asterisk) and both LV papillary muscles have LGE. In one view, it is not possible to clearly ascertain whether other faint enhancement is all due to the tricuspid valve. (b) Shows the VSD patch site scar (asterisk) and RVOT scar (solid arrow) as well as LGE in the left ventricle (LV) apex consistent with apical vent. (c and d) Show VSD patch scar (asterisk), small-degree RVOT scarring (solid arrow) and scarring in RV trabeculation (dotted arrow) confirmed in another RV in and out images in systole and after phase swap. This small LGE focus is also seen in image (a) (dotted arrow)
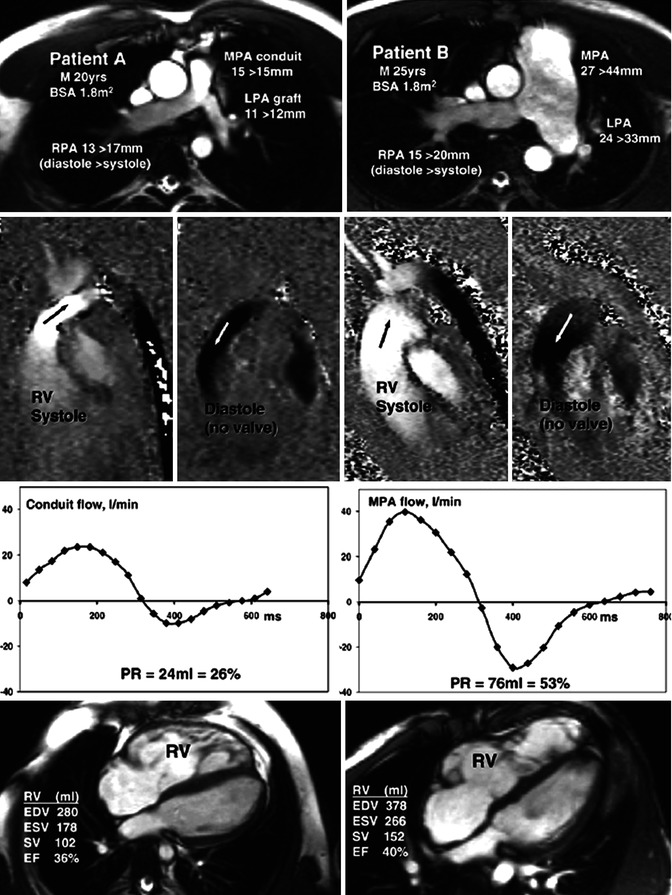
Fig. 9.6
Differing pulmonary regurgitation fraction with ineffective pulmonary valve in TOF. CMR studies in two patients, neither with an effective pulmonary valve but with different amounts of pulmonary regurgitation. Patient A (left panels) with TOF was repaired using a right ventricle (RV) to pulmonary artery (PA) homograft conduit with graft augmentation of the proximal LPA. Patient B (right panels) with similar body surface area, born with pulmonary and subpulmonary stenosis and a ventricular septal defect, had patch reconstruction of the RVOT aged 2 years. Cine imaging (upper row) showed marked differences of size and expansion of the proximal pulmonary arteries, replaced by a conduit and graft in patient A but dilated and expansile in patient B, as indicated by the end-diastolic peak systolic diameter measurements shown. In-plane, vertically encoded velocity maps aligned with the RV outflow tract (second row) show no effective valve action in diastole. Flow curves (third row) were plotted from retrospectively gated acquisitions of velocities through planes transecting the proximal main PA or conduit, giving the regurgitant volumes and fractions. The peak systolic velocity was 3 m/s in the conduit of patient A and 1 m/s in the main PA of patient B. Patient A had a higher heart rate and a less dilated RV as shown by the four-chamber cines and right ventricular volume measurements (bottom row). M male, BSA body surface area, MPA main pulmonary artery, RPA right pulmonary artery, LPA left pulmonary artery, RV right ventricle, PR pulmonary regurgitation, EDV end-diastolic volume, ESV end-systolic volume, SV stroke volume, EF ejection fraction (From Kilner et al. [14] with permission)
Image acquisition in TOF should include:
1.
Multislice stacks in transaxial, coronal and sagittal planes (with HASTE or single-shot bSSFP).
2.
Cines of two-chamber, four-chamber and short-axis stacks; two views each of left and right (Fig. 9.1) ventricular outflow tracts, aortic valve views should be acquired for further evaluation of aortic root. Dedicated right pulmonary artery (RPA) and left pulmonary artery (LPA) views (cines and velocity mapping) should be obtained if there is suspicion of branch pulmonary artery obstruction. In our experience, long-axis oblique RV views showing the aorta in short axis, pulmonary valve and tricuspid valve which shows the RV outflow tract (‘RV in and out’), a similar image plane with the aorta in long axis orientated more towards the body and apex of the RV (‘RV oblique’) and two-chamber right atrial right ventricular views perpendicular in orientation to the four-chamber view are useful for qualitative, regional RV assessment (Fig. 9.2). Additional ‘cross-cut’ cines may be required in the presence of tricuspid regurgitation or VSD to better delineate these lesions.
3.
Phase–shift velocity mapping to derive pulmonary regurgitation fraction. Flow mapping for pulmonary regurgitation should be aligned perpendicular to the pulmonary trunk in at least two cine RVOT views. In–plane RVOT velocity mapping is not only useful to locate a subsequent throughplane velocity map appropriately for determination of peak pulmonary velocity but also useful to align the throughplane mapping for pulmonary regurgitation. A subpulmonary valve level throughplane velocity map is useful to demonstrate the size of the regurgitant jet. To determine cardiac output as well as identify any residual intracardiac shunt by calculation of Qp:Qs, an aortic throughplane velocity map should be acquired at the aortic sinotubular junction. Where appearances suggest potential branch PA stenosis, right and left PA throughplane velocity mapping should be performed to assess preferential branch PA blood flow (Fig. 9.3). In unilateral pulmonary stenosis, this helps assess severity. Normal differential pulmonary blood flow is RPA:LPA 60 %:40 %.
Specific Considerations for CMR Reporting in TOF
Pulmonary Regurgitation and Other Valvar Assessments
CMR plays an important role in the assessment of pulmonary regurgitation, which is common two to three decades after repair of TOF (Fig. 9.6). Pulmonary regurgitation is implicated in progressive RV dysfunction, arrhythmia and sudden cardiac death. Free pulmonary regurgitation is whereby there is no effective valvar function apparent both with anatomical features on cine imaging and when forward and reverse flow jets on in-plane velocity mapping are equal in diameter. Free pulmonary regurgitation may be tolerated without symptoms for decades and is typically associated with a regurgitant fraction of approximately 30–40 % (in the absence of additional distal branch or peripheral PA stenosis). This discrepancy with aortic regurgitation assessment is for two reasons. Firstly, as in the Fontan circulation, forward pulmonary flow can occur in the absence of an RV due to the effects of inspiration and also because the left ventricle ejects blood out of the thoracic cavity creating a negative pressure that sucks blood into the PA. Secondly, the pulmonary microvascular resistance is low and situated relatively near to the heart. As a result RV systole moves blood through the pulmonary microcirculation including capillaries into the low pressure pulmonary veins. Flow that passes through alveolar capillaries does not pass back again in diastole as there is no significant reversal of gradient. Thus, the magnitude of the regurgitant fraction is limited unless there are additional factors such as branch PA stenosis which may increase regurgitation leading to progressive RV dysfunction [13]. The calculated pulmonary regurgitant fraction may be lower despite ineffective valve function if there is valvar or subvalvar RVOT obstruction or an incompliant RV but higher if there is distal pulmonary stenosis and pulmonary hypertension (rare in this condition but could be present related to, e.g. a previous Waterston shunt) or with increased pulmonary arterial compliance [14]. Arguably, pulmonary regurgitant volume may be more relevant [15]. Pulmonary regurgitation also varies with respiration [16]. Serial scans allow monitoring of the severity of pulmonary regurgitation in tandem with the effects of chronic volume overload on biventricular haemodynamics and function. Once pulmonary regurgitation is moderate or severe, clinical decision-making with regard to timing of pulmonary valve implantation is influenced by RV volumes. PVR improves biventricular mechanics and patient symptoms (Fig. 9.7).
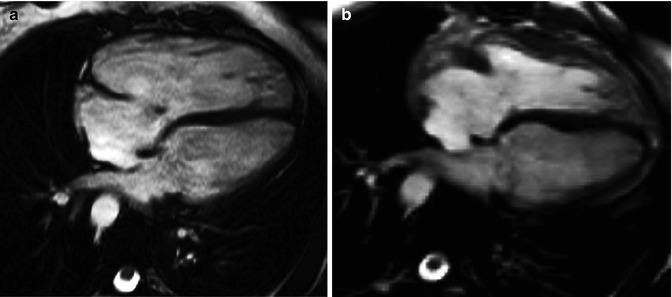

Fig. 9.7
Effect of pulmonary valve regurgitation on biventricular mechanics. Diastolic frame from four-chamber cine preceding pulmonary valve replacement for pulmonary regurgitation in the setting of TOF (a) compared with diastolic four-chamber cine after pulmonary valve replacement (b). In image b versus a, the right ventricle can be seen to have reduced in size, there is no longer a small jet of tricuspid regurgitation, and left ventricular diastolic filling appears to have increased
Tricuspid regurgitation may be found in association with dilatation of the RV related to pulmonary regurgitation and less commonly in isolation due to damage at surgery or congenitally dysplastic tricuspid valve. Dedicated cross-cuts of jets on cines will be needed, and the degree of regurgitation can be assessed from a throughplane flow image of the regurgitant jet and from tricuspid regurgitant fraction (difference between RV stroke volume and forward flow in the PA at pulmonary trunk velocity mapping divided by total RV stroke volume and expressed as a percentage). Aortic regurgitation may be associated with aortic root dilatation and can be quantified by aortic regurgitant fraction calculated from velocity mapping located at the sinotubular junction. Mitral regurgitation is rare.
Global and Regional Ventricular Function Assessment and Ventricular–Ventricular Interaction
CMR makes a very valuable assessment of both right and left ventricular function. Geometrical assumptions that are relied upon using echocardiography are totally unsuitable to the tripartite RV that unlike the left does not approximate an ellipsoid shape. Furthermore, in TOF the RV is yet more complex due to alterations from surgical intervention. Volumes, mass and ejection fraction for the RV are not encumbered by geometrical assumptions when using CMR for analysis. Therefore, for volumetric function quantification, CMR is a gold standard for the RV. As discussed below, it is also highly suitable to assessment for regional RV abnormality most commonly affecting the outflow tract in TOF. There is interest in so-called ventricular interaction in TOF. Certainly RV volumes and function relate to LV volumes and function in this condition [17, 18], which may be explained by a number of factors, some of which are perhaps unsurprising. These include shared pericardium, a parallel circulation, a shared septum and interdigitation of the RV and LV myocardial fibres. It seems that in the late follow-up of repaired TOF, LV dysfunction may be a consequence of RV dysfunction.
Global Assessment of Ventricular Volumes, Mass and Function
There is no doubt that CMR is the gold standard technique for right as well as left ventricular volume assessment and no other technique has as excellent a reproducibility. This quality of CMR is the reason fewer patients are needed to power clinical trials [19]. However, there are important differences in approaches and measurements made by different operators across various institutions. Awareness of these is important to determine the applicability of published literature to the individual patient in an individual institution. Defining the valve planes may be ambiguous. The pulmonary valve may be absent or just a small remnant. The basal short-axis slice may include right atrial tissue even in systole when the RV is dilated. In the context of a heavily trabeculated ventricle, the volumes may be operator dependent. Whether the approach chosen is a transaxial ventricular volume stack or short axis and whether the trabeculations are excluded from the blood pool or ignored, each operator and centre requires a robust and reproducible protocol. A transaxial stack approach has been argued for because some operators have found it more reproducible and certainly it may be easier to determine the tricuspid valve plane. An argument in favour of a short-axis approach is that TOF is a biventricular disease. LV dysfunction is an important predictor of mortality, though it is likely to develop late and partly as a consequence of RV dysfunction. Given right as well as left ventricular volumes are valuable, having an approach that may be easier for the RV but requiring the LV to be measured at a different time with a different data set adds time and biological variability to the CMR study. In TOF, RV assessment is as pertinent as the left but poses challenges in accurately assessing the thin myocardium and coarse trabeculations, which increase towards the apex. These complex trabeculations increase in the presence of RV hypertrophy and can be difficult to visualise and outline individually. Ignoring RV trabeculations may be a more rapid and reproducible way [20] to approach ventricular volume assessment. However, not only may this be inaccurate for RV mass measurement but as others have also stated, this leads to incorrectly large volume estimates for the RV and prohibits the internal validation of stroke volumes through comparison with aortic and pulmonary flow volumes [21]. Our own practice is to exclude trabeculations from the blood pool (Fig. 9.8). Careful demarcation of the borders of the RV can give highly reproducible results even in the context of complex operated RVs in TOF. [22] Whilst reproducible with a single experienced operator, this method is labour intensive and requires at least 30 min per patient. However, for research, this degree of reproducibility enabled adequate powering of a randomised controlled trial in repaired TOF with chronic pulmonary regurgitation to assess the effects of ramipril (the APPROPRIATE study) [23].
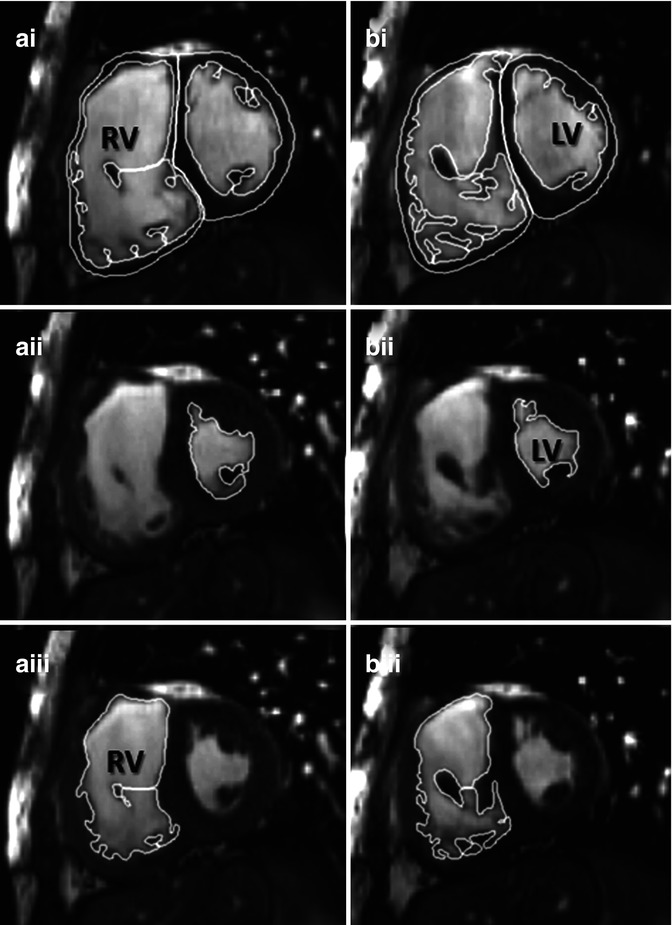

Fig. 9.8
Example of contouring right ventricle (RV) volumes in TOF. Manual planimetry of short-axis contiguous slices from base to apex of the heart has proven highly reproducible for research in our centre. Trabeculations will be excluded from the blood pool and included in the ventricular mass. In this example at levels a and b, RV end systole (aiii and biii) was in a different frame from left ventricular end systole (aii and bii) consistent with the patient having wide right bundle branch block. End diastole (ai and bi) for both ventricles are synchronous. LV left ventricle
Consistency between serial measurements should be optimised through standardisation of scanning approaches and protocols and regular quality assurance. Practically, because intraobserver variability is lower [22], it may be important for a single operator to remeasure a previous CMR scan at the time of a current report to be certain if there is true change in RV ventricular volumes. Awareness of technical issues regarding acquisition and analysis is important as RV volume measurements are pivotal to clinical decision-making regarding the indications for pulmonary valve implantation in asymptomatic patients and assessment of the haemodynamic benefits of surgery.
Numerous contributions have been made to the literature in the last 10 years describing the value of CMR RV volume assessment for determining indications for pulmonary valve replacement [24–32] though still more data are needed to determine optimal timing in asymptomatic TOF patients. Oosterhof et al. have implied surgical PVR should be carried out before RV end-diastolic volume exceeds 160 mL/m2 or RV end-systolic volume >82 mL/m2 as these thresholds predict return of RV volumes to normal size following PVR [30]. Another group found RV end-systolic volume <90 mL/m2 to be a predictor of normal RV size and function postoperatively [33]. The implication is that beyond this degree of RV dilatation, there is limited reversibility of RV dilatation and dysfunction. Other authors suggest PVR might be indicated even earlier before RV end-diastolic volume exceeds 150 mL/m2 [28, 31]. In a number of centres, an RV:LV diastolic volume ratio of ≥2:1 is also considered significant. Consensus has been established amongst ACHD clinicians that the previous clinical management of operating on repaired TOF patients later when symptoms are established is too late.
European Society of Cardiology (ESC) Guidelines for ACHD [34] advocate pulmonary valve replacement in symptomatic TOF patients with severe pulmonary regurgitation and/or stenosis with RV systolic pressure >60 mmHg/TR velocity >3.5 m/s with a Class 1 indication. In asymptomatic patients, Class IIa indications for pulmonary valve intervention in the presence of severe pulmonary regurgitation and/or stenosis are:
An objective decrease in exercise capacity has been demonstrated.
Progressive RV dilatation on CMR.
Progressive RV systolic dysfunction.
Progressive (at least moderate) tricuspid regurgitation.
RVOT obstruction with RV systolic pressure >80 mmHg/TR velocity >4.3 m/s.
Sustained atrial and/or ventricular arrhythmias.
These guidelines are not prescriptive with regard to nonserial CMR measures. In our centre RV:LV ratio ≥2:1 with RVEDVi >150 mL/m2 usually prompts multidisciplinary discussion of elective pulmonary valve implantation which is an individualised decision based also on non-CMR-based data such as objectively measured exercise capacity.
Regional Assessment of the RV Including Akinetic Outflow Tract Regions
In older patients, reflecting the earlier era of TOF repair, akinetic and or aneurismal areas in the RVOT are common and present even in the absence of patch reconstruction of the RVOT [17] (Fig. 9.9). Given the limits of the RV are the tricuspid and pulmonary valves, any akinetic or aneurismal region in the RVOT is included in the ventricular volumes. Such akinetic and or aneurismal regions are adverse not only as they relate to RV systolic dysfunction [17] but also because they cause conduction delay within the RV contributing to prolonged QRS duration [35] and are associated with scar [36, 37]. These dilated or dyskinetic RVOT regions therefore lower the calculated ejection fraction as they are included in the ventricular volume. The disadvantage of including them is that small progressive reductions in function of the contractile RV beneath the akinetic area may be masked. A reproducible method to characterise the function of these regions separately is, however, not widely available. We report a simple linear measurement based on the sagittal RVOT view. We use this feasible measure to help describe the relative contribution of outflow tract pathology to impaired RV ejection fraction and to describe size. Recently a prospective follow-up study for clinical outcomes demonstrated that larger akinetic area length predicted sustained ventricular arrhythmia [38]. Routine views such as four-chamber cine may be misleadingly normal, whereas long-axis views (RV outflow tract, LV outflow tract, oblique RV views) are more likely to show wall thinning and regional motion abnormality because the primary pathology is of the RV infundibulum.
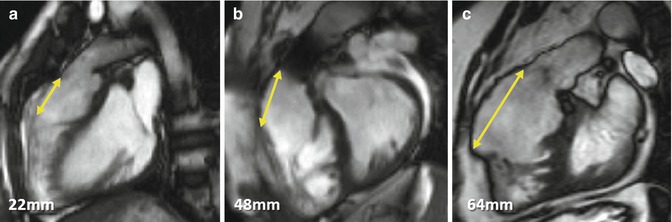

Fig. 9.9
Right ventricle outflow tract (RVOT) akinetic area in TOF. Examples of simple linear RVOT measurements in sagittal RVOT planes in TOF patients A, B and C are shown. These measurements should be taken after also reviewing the left ventricular outflow tract plane as well as the short-axis stack. Not all akinetic areas are necessarily associated with RVOT patching and muscle bundle resection alone can result in thinning and regional wall motion abnormality. Artefact in the pulmonary trunk of patient B is due to previous percutaneous pulmonary valve implantation
Global and Regional LV Dysfunction
LV regional wall motion abnormality may be present in TOF. A small area (<1 cm) at the apex may be present and related to apical vent insertion at the time of surgery in order to deair the heart. However, more extensive abnormalities have been noted and associated with scarring either in small localised areas or in a larger anteroapical distribution [36




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree