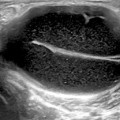
Fig. 3.1
Longitudinal view over the midline dorsal aspect of the wrist showing B-mode synovitis (a) with Doppler signal (b) in radio-carpal (asterix) and midcarpal (star) joints; r radius, c capitate
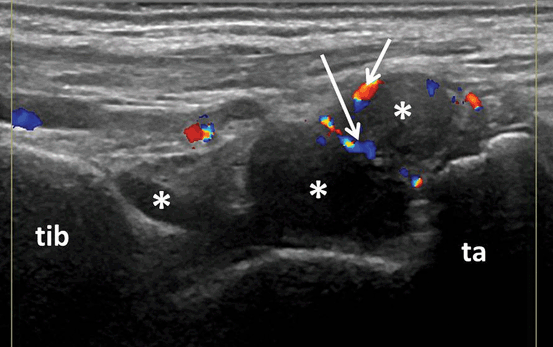
Fig. 3.2
Longitudinal view of the dorsal aspect of the tibio-talar joint showing B-mode synovitis (asterix) with Doppler signal (arrows); tib tibia, ta talus
While knee arthroscopy, a frequent procedure in clinical practice, has permitted a relatively easier histopathological assessment of inflamed joints, MSUS, both BM and Doppler, were reported to be accurate in detecting joint synovitis in comparison to arthroscopy and histology, respectively [7, 24]. Earlier studies reported good correlation between histological and B-mode ultrasound (BMUS) findings in knee joint synovitis. Furthermore, in patients with knee joint involvement in different diseases, BMUS has shown a high sensitivity, specificity, and accuracy (Fig. 3.3) for detecting synovitis [7, 24]. In RA inflamed joints, there was a similar good correlation between histologic and Doppler inflammatory changes in different joints [25–27]. When comparing histopathology with BMUS, power Doppler ultrasound (PDUS), and MRI, the highest correlation was found for PDUS and histopathology [28]. Although false-negative results were found for Doppler techniques when compared to histology [27, 29], the presence of a positive Doppler signal in the synovium was an indicator of active synovial inflammation.
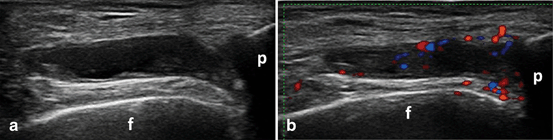

Fig. 3.3
Transverse view over the parapatellar recess of the knee showing B-mode synovitis (a) with Doppler signal (b) synovitis; f femur, p patella
When using others imaging techniques as comparator, MSUS, both BM and Doppler, showed considerable sensitivity and specificity. In a number of studies, moderate to good correlations were found between US-detected synovitis (either BM or Doppler) and MRI-detected synovitis in hand finger joints [12, 30]. Using MRI as reference, Szkudlarek et al. found a good to excellent sensitivity and specificity of US, both BM and PD, for the detection of synovitis at metatarsophalangeal (MTP) and metacarpophalangeal (MCP) joints [31, 32]. Similarly, Scheel et al. reported a good agreement between US and MRI in the detection of BM synovitis at MCP and proximal interphalangeal (PIP) joints [33]. In concordance with these data, Terslev et al. depicted a high significant association between Doppler US indices of inflammation and post-contrast MRI scores at wrist and hand joints [34]; whereas Fukae et al. found a good correlation between the measurements of Doppler synovitis and the enhancement rate of MRI in MCP and PIP joints [30]. Recently, Kawashiri et al. observed a moderate to good correlation between US-detected synovitis (in both BM and PD) and MRI-detected osteitis [35] (Fig. 3.4) .
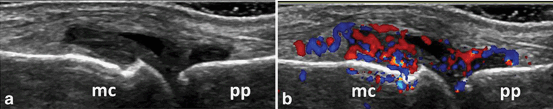

Fig. 3.4
Longitudinal view of the dorsal aspect of the metacarpophalangeal joint showing B-mode synovitis (a) with Doppler signal (b); mc metacarpal head; pp proximal phalanx
Good correlation was also reported in other studies investigating the link between MSUS-detected synovitis and inflammation identified through clinical examination and laboratory analysis. Naredo et al. found a moderate to good correlation between swelling joints count and MSUS-detected synovitis for both BM and PD. MSUS-detected synovitis was also found to better correlate with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) than clinically detected synovitis [10]. In the study by Scire et al. on patients with early RA who started csDMARDs treatment, both clinical and MSUS-detected synovitis which were significantly correlated with CRP in patients with active disease, while, in patients who achieved the clinical remission status, only PD correlated with CRP [14]. Kawashiri et al. found a significant moderate to good correlation between PDUS assessment of 12 joints with serum vascular endothelial growth factor (VEGF), matrix metalloprotease (MMP)-3, and metallopeptidase inhibitor (TIMP)-1 [36] .
Studies that have investigated intra- and interobserver reliability in a variety of joints for MSUS-detected synovitis, for both BM and Doppler showed moderate to excellent results [7, 10, 13, 30, 37–39]. A systematic review assessing the reliability of MSUS-detected synovitis in RA showed that US, particularly PD mode, was reliable in still-image interpretation when assessed by experienced ultrasonographers, while image acquisition was less reliable. Among all joints, the knee was the most reliable joint even in terms of image acquisition [40]. Mandel et al. compared the reliability of 11 different US scoring systems for synovitis including different combinations of joint counts [42, 28, 20, 16, 12, 10, 8, 7] and found that MSUS, both BM and PD, showed a better reliability than clinical assessment in evaluating synovitis. No differences in the reliability were observed between these scoring systems [41].
The sensitivity to change of US-detected synovitis has been investigated in several published studies. Regardless of how many joints were evaluated, a decrease of BM and Doppler variables has been shown in patients treated with bDMARDs [15, 42–46] or csDMARDs [47, 48]. In a randomized control trial, Taylor et al. studied patients with early RA treated with methotrexate (MTX) and infliximab (IFX) versus MTX and placebo using US and conventional radiography (CR) for the evaluation of MCP joints. After 18 weeks of treatment, patients under IFX therapy presented significant reduction in synovial thickness and joint vascularity measured as the number of CD pixels in a defined region of interest [49]. Responsiveness of US-detected synovitis has also been shown after steroid treatment, either intra-articular or systemic [50–54] .
Several scoring systems have been developed to assess synovitis, namely SH, SE, and inflammatory activity by Doppler US. Among them, semiquantitative scores have been the most used scores in clinical practice [18, 46]. In most published studies of MSUS in RA, the semiquantitative score for BM synovitis consisted of the following: grade 0—absence of synovial thickening, grade 1—mild synovial thickening, grade 2—moderate synovial thickening, grade 3—marked synovial thickening [18]. Similarly, in most published studies, the semiquantitative score for Doppler synovitis consisted of the following: grade 0—no flow in the synovium, grade 1—up to three single spots signals or up to two confluent spots or one confluent spot plus up to two single spots, grade 2—vessel signals in less than half of the area of the synovium, grade 3—vessel signals in more than half of the area of the synovium [18, 46]. Quantitative measurements of Doppler signals are obtained using a color recognition function that counts the number of total and color pixels within a region of interest. The number of color pixels is then expressed in relation to the total number of pixels as a fraction [44, 51, 55]. Terslev et al. found a good agreement between quantitative and semiquantitative scores for Doppler synovitis [56] .
While small joints can be more easily assessed by clinical examination, synovitis evaluation of larger joints mostly shoulders and hips, represents a challenge in daily clinical practice. In a recent study, Sakellariou et al. found inflammatory changes at glenohumeral (GH) joint in 14 % of RA studied patients [57]. Figure 3.5 shows an example of posterior GH recess showing BM synovitis with Doppler signal in an RA patient (Fig. 3.5). Even if it is not so frequently evaluated, hip involvement is not uncommon in RA patients. In an Italian cohort of RA patients, BMUS detected hip synovitis in 24 % of patients [58]. Figure 3.6 shows an example of the anterior recess of the hip showing BM synovitis. However, Doppler activity is not detected so frequently in these joints, due to the lower sensitivity of Doppler techniques for deep areas .
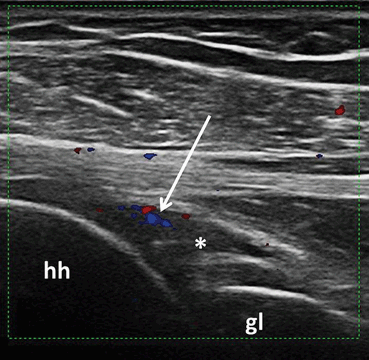
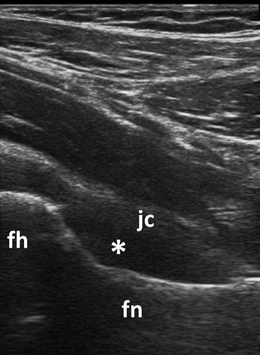

Fig. 3.5
Longitudinal view over the posterior glenohumeral recess showing B-mode synovitis (asterix) with Doppler signal (arrow); hh humeral head, gl glenoid fossa

Fig. 3.6
Longitudinal view to the femoral neck showing B-mode synovitis (asterix) in the anterior recess of the hip; fh femoral head, fn femoral neck, jc joint capsule
Tenosynovitis is another important feature in RA patients. US-detected tenosynovitis is defined as hypoechoic or anechoic thickened tissue with or without fluid within the tendon sheath, which is seen in two perpendicular planes (Fig. 3.7) and which may exhibit Doppler signal [17]. Tenosynovitis on Doppler mode is defined as the presence of peri-tendinous Doppler signal within the synovial sheath (Fig. 3.8), seen in two perpendicular planes, excluding normal nutrient vessels in mesotenon or vinculae, only if the tendon shows peri-tendinous synovial sheath widening on BM [37].
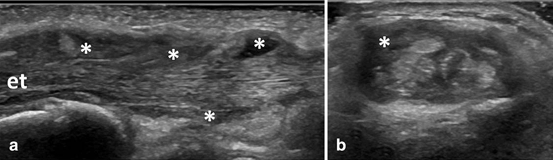
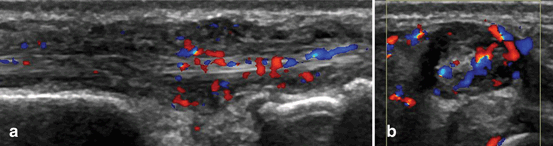

Fig. 3.7
Longitudinal (a) and transversal (b) view over the second compartment of the wrist extensor tendons showing B-mode tenosynovitis (asterix); et extensor tendons

Fig. 3.8
Longitudinal (a) and transversal (b) view over the extensor carpi ulnaris tendon showing B-mode and Doppler tenosynovitis
Compared to MRI, MSUS has shown to be accurate in detection of tenosynovitis. MSUS has also shown a high specificity, but a fair to moderate sensitivity for detecting tenosynovitis [21]. A number of studies have compared US and MRI evaluation of tenosynovitis. In the study by Hoving et al., MSUS detected more tendon effusion than MRI at wrist and hand tendons [59]. Scheel et al. found a good agreement between US and MRI for tenosynovitis at ankle flexors and peroneus tendons while for ankle extensor tendons they reported a lower agreement [11].
Regarding the intra-observer consistency of US-assessed tenosynovitis, various studies have shown good to excellent reliability [19–21] and moderate to excellent interobserver reliability [19, 20, 22, 47]. Hammer et al. studied the sensitivity to change of US-detected tenosynovitis in RA patients initiating adalimumab (ADA) treatment. They assessed flexor and extensor tendons of bilateral wrist and ankle and observed a significant reduction of tenosynovitis after 12 months for all studied tendons. The MSUS assessment of a reduced number of tendons (i.e., extensor carpi ulnaris (ECU), tibialis posterior (TP), and flexor digitorum longus) was as sensitive to change as the assessment of all studied tendons [60] .
For tenosynovitis, the most studied tendons have been the hand and ankle tendons. However, in RA not all tendons are affected in the same way. At hand and finger level, the most frequently involved tendons are the ECU and the flexor tendons of the second, third, and fourth fingers [61]. In an MRI study of RA patients with hindfoot pain, the most frequently involved tendons were TP and peroneal tendons and the least common involved tendons were tibialis anterior (TA) and the extensor tendons [62]. Figure 3.9 shows an example of BM tenosynovitis of TP tendon in an RA patient.
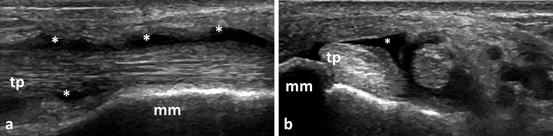

Fig. 3.9
Longitudinal (a) and transversal (b) view over tibialis posterior tendon showing B-mode tenosynovitis (asterix); tp tibialis posterior, mm medial malleolus
Recently, the OMERACT MSUS group developed a four-grade semiquantitative scoring system for BM and Doppler tenosynovitis which showed a good intra- and interobserver reliability [19]. This score is as follows: grade 0—normal, grade 1—minimal, grade 2—moderate, and grade 3—severe. Doppler tenosynovitis was scored as following: grade 0—no Doppler signal, grade 1—minimal, grade 2—moderate, and grade 3—severe pathological peri-tendinous Doppler signal within the synovial sheath [19] .
Bone erosions are defined, according to OMERACT, as intra-articular discontinuity of the bone surface that is visible in two perpendicular planes (Fig. 3.10) [17].
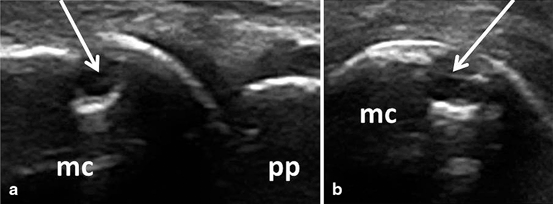

Fig. 3.10
Longitudinal (a) and transversal (b) view of the dorsal aspect of the metacarpophalangeal joint showing an erosion (arrow); mc metacarpal head, pp proximal phalanx
Over the past decades, CR has been the primary choice in assessing bone erosions. However, early in the disease course, CR cannot always detect bone changes. MSUS was shown to be more sensitive than CR in detecting bone erosions at finger and toe joint level [12, 31, 63–65]. This was supported by various studies which revealed a high agreement between US and MRI in detecting bone erosion at hand and foot finger joints [12, 31]. At the hand finger joint level, the agreement was higher in joints with good accessibility for US, like second and fifth MCP [12]. Finzel et al. reported a good correlation between the severity of erosions detected by US and by micro-computed tomography (micro-CT) [66]. Furthermore, using MRI and CT as reference, US has shown a high specificity with a moderate sensitivity in detecting bone erosions [12, 67]. However, sensitivity improved considerably, without losing specificity, when only US-accessible areas, i.e., radial second MCP, ulnar fifth MCP, and all dorsal/palmar aspects, were included [67]. In another study, Døhn et al. compared US- and MRI-detected erosions with CT-detected erosions at MCP joints in RA patients without CR-detected erosions. With CT as reference, US and MRI resulted in high specificity in detecting bone erosions even in normal radiographic MCP joints [68]. On the other hand, when compared to CT, false-positive results for US-detected erosions could be noted, especially in small joints. This is mostly due to the misinterpretation of normal vascular bone channels and normal grooves of the metacarpal heads that can mimic bone erosions [66] .
On another front, MSUS has shown a good reliability for detecting bone erosions. Several studies revealed good to excellent intra- and interobserver reliability for both small and large joints [18, 39, 63, 69]. For small joints, the highest agreement was found at second MCP joint [18].
In published studies on RA patients, the most commonly used score for bone erosions has been semiquantitative scores. There are a number of different semiquantitative scores for bone erosion used in these studies. Szkudlarek et al. proposed a semiquantitative scoring from 0 to 3 (0—regular bone surface, 1—irregularity of the bone surface without formation of a defect seen in two planes, 2—formation of a defect in the surface of the bone seen in two planes, 3—bone defect creating extensive bone destruction) system that demonstrated a good intra-observer agreement [18]. Another semiquantitative scoring system was proposed by Wakefiel et al. based on the size of erosions (small erosion ≤ 2 mm, moderate erosion 2–4 mm, and large erosion ≥ 4 mm), that also showed good intra- and interobserver agreement [63] .
The most frequent site for MSUS-detected erosions in RA patients at hand level are the second MCP and fifth MCP joints, and at foot level are the first MTP and fifth MTP joints, while the fewest erosions are detected at fourth MCP joint [63, 64, 70, 71]. An explanation for these findings could be the acoustic window for US beams at this level. The majority of erosions were detected at the metacarpal heads and on the radial or ulnar sites of the joints, while lesser erosions were detected at the phalangeal bases and on the dorsal and volar aspect of the joints [63]. In contrast, at wrist level, US evaluation of erosions is difficult due to the irregularities of the bone margins, the presence of several nutrition channels, and poor US-window for structure visualization [72]. Humeral head erosions can be seen in a significant number of healthy people [73]. Thus, clinical conclusion cannot be drawn from US-detected erosion at this level, especially for small erosions.
Tendon damage is a common finding in long-standing RA patients as repeated or persistent tendon inflammation can lead to structural damage and tendon rupture. Compared to MRI, US is seemingly more sensitive in detecting partial finger extensor tendon tear [74]. However, until recently, there was no commonly agreed definition or scoring system for tendon damage. In 2013, the OMERACT MSUS group defined tendon damage on BM as internal and/or peripheral focal tendon defect (i.e., absence of fibers) in the region enclosed by tendon sheath (Fig. 3.11), seen in two perpendicular planes [75] . For tendon damage, a three-grade semiquantitative scoring system has recently been developed (grade 0—normal, grade 1—partial, and grade 3—complete rupture). This scoring system resulted in good interobserver agreement and excellent intra-observer agreement [75]. Good to excellent intra- and interobserver agreement was found in various studies for tendon damage at hand and ankle tendons [20, 22].
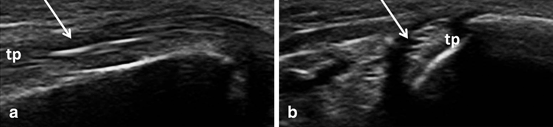

Fig. 3.11
Longitudinal (a) and transversal (b) view over tibialis posterior tendon showing tendon damage (arrow); tp tibialis posterior, mm medial malleolus
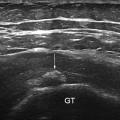
Bursitis Inflammation of periarticular soft tissue, including synovial bursae, is a major cause of pain in RA patients. Accurate diagnosis of such pathologies is of utmost importance for adequate management of these patients. The most studied bursal sites are at the shoulder and foot level. Figure 3.12 shows an example of subacromial subdeltoid (SASD) bursitis in an RA patient. In a study carried out by Bruyn et al., they reported a good overall agreement between US and MRI in detecting SASD bursitis. In the same study, intra- and interobserver reliability for BM and PD ranged from poor to moderate [76]. At forefoot bursitis, the most frequently involved site was the 4/5 inter-metatarsal space [77] .
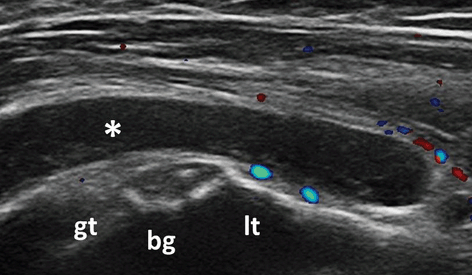

Fig. 3.12
Transversal view over the bicipital grove showing subacromial subdeltoid bursitis (asterix); Doppler signal is seen outside the bursa; gt greater tuberosity, lt lesser tuberosity, bg bicipital groove
An important cause of knee pain is the presence of Baker’s cyst. Figure 3.13 shows an example of Baker cyst showing SH with Doppler signal and SE in an RA patient. Diagnosis is essential as treatment is different from other knee pathologies. In a number of studies, almost a half of RA patients assessed had US-detected Baker’s cyst [8, 78, 79] of which less than a half were detected by clinical examination [78].
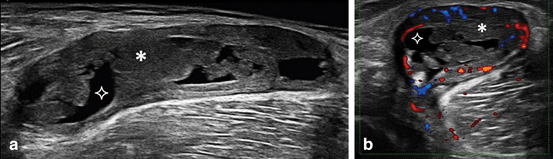

Fig. 3.13
Longitudinal extended (a) and transversal (b) view over a Baker’s cyst showing synovial hypertrophy (asterix) with Doppler signal and synovial effusion (star)
Enthesopathy was defined as abnormal hypoechoic (loss of fibrillar architecture) and/or thickened tendon at its bony attachment seen in two perpendicular planes that may exhibit Doppler signal and/or bony changes including enthesophytes, erosions, or irregularity [17]. Although entheseal abnormalities in RA patients are insufficiently studied, it seems that these are more frequent than has been previously estimated. Genc et al. compared tendon and entheseal US abnormalities of lower and upper limb in RA patients with spondyloarthropathy (SpA) and healthy controls. They found that there were no significant differences between RA and SpA patients in terms of tendon and entheseal involvement, whereas RA patients presented more tendon and entheseal pathologies than healthy controls. The most affected entheseal sites in RA and SpA patients were the distal and proximal patellar tendon and Achilles tendon. No differences from the control group were found in the involvement of plantar aponeurosis [80] .
Rheumatoid nodules are more frequently found at pressure sites, usually associated with more severe disease. At US examinations, they appear oval shaped, with well-defined hypoechoic formation, generally homogenous, and in the majority of cases they are usually found close to the bone surface. They can present a central very hypoechoic, well-defined area. Compared to gout tophi, rheumatoid nodules show less frequent posterior acoustic shadowing and less erosion at adjacent bone level [81] .
Clinical Applications
Besides demonstrating to be a valid, reliable, and sensitive-to-change tool in inflammatory arthritis , MSUS has also been shown to be more sensitive than clinical assessment in detecting joint inflammation in RA patients. Irrespective of the number of joints studied, disease activity, or duration, MSUS has detected inflammation in significantly more joints than clinical assessment [7–16, 39].
Currently, the main role of US assessment in RA includes diagnosis, monitoring disease activity and treatment response as well as guiding intra-articular procedures.
Diagnosis
Although several joint abnormalities can be detected by MSUS, none of them are pathognomonic for RA. However, a number of studies have shown that MSUS can be used for diagnostic purposes in addition to clinical evaluation [82, 83], especially in seronegative patients [84]. Noteworthy US findings were not interpreted out of clinical context. For the diagnostic purposes, the majority of studies have investigated the added value of US-detected abnormalities at small joint level (i.e., MCP, PIP, MTP wrist and ankle joints). Early US-detected abnormalities at this level were mostly synovitis and tenosynovitis, although erosion detection was not uncommon. Results of these studies paved the way for EULAR recommendations regarding the use of US when diagnostic doubts arise, as this would improve the certainty of an RA diagnosis above clinical criteria alone [23].
For BM synovitis, there is no consensus regarding the relevance of grade 1 of synovitis, especially in small joints. BM synovitis grade 1 can be detected in a significant percentage of healthy people, and at least for diagnosis purposes its use is debatable [85]. Figure 3.14 shows an example of grade 1 BM synovitis in healthy people. Although the presence of intra-articular Doppler signals is associated more frequently with pathology, it can be detected also in healthy people [86]. This is possible mainly due to the improvement of machines sensitivity which allows the detection of normal vessels. Thus, the sensitivity of the machine must be considered and settings adjusted accordingly. The presence of grade 1 synovitis, especially in one isolated joint, without other inflammatory changes should be carefully considered as diagnosis value. Noteworthy, all the above remarks are valid when assessing patients without any anti-inflammatory treatment, as this can mask the BM synovitis and Doppler activity. Also synovitis, erosions can also be seen in healthy people [31, 68, 87].
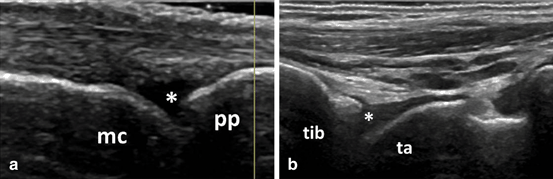

Fig. 3.14
Images showing B-mode synovitis grade 1 (asterix) at metacarpophalangeal joint (a) and tibiotalar joint (b) in healthy people; mc metacarpal head, pp proximal phalanx, tib tibia, ta talus
The minimal threshold enough to diagnose active inflammatory arthritis remains a matter of controversy. This may include the minimal degree of synovitis, number of joints with synovitis, degree of erosions, or a combination of any three that are necessary to make an RA diagnosis. Millet et al. suggested a minimum two joints showing grade 2 or 3 for BM-detected synovitis or two cases of bone erosion [87]. Other studies added tendon evaluation to US assessment of inflammatory arthritis to make an RA diagnosis. These US findings—together with clinical and laboratory findings—increase the probability of an inflammatory arthritis diagnosis. In the study by Freeston et al., US evaluation of wrists and MCP joints and flexor tendons was added to clinical examination in patients with very early inflammatory arthritis. In seronegative patients with positive CRP, swollen joints and erosion on CR, the presence of a grade 3 BM synovitis, at least a grade 1of PD synovitis, or at least one erosion increases the probability of inflammatory arthritis from 30 to 94 % [84]. In a study of early, untreated oligoarthritis, following US assessment, about one third of patients fulfilled polyarthritis classification criteria owing to the presence of subclinical synovitis [9]. According to a study by Scire et al., a PD score of two or more was highly specific for the diagnosis of RA [88]. Thus, the tendon evaluation can add valuable information about inflammatory activity. Furthermore, it is important to remember that a number of the tendons’ synovial sheaths can communicate to the synovial joint (e.g., biceps tendon sheath, foot first finger flexor tendon).
In early, untreated RA patients, finger flexor tenosynovitis was observed more frequently than peri-extensor tenosynovitis (Fig. 3.15), and the most frequently involved tendons were the tendons from second and third fingers [21]. In an MRI study, hand flexor tenosynovitis was a strong predictor for RA in early, unspecified arthritis or suspected RA [89].
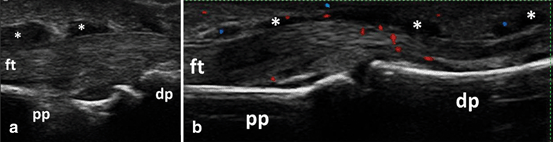

Fig. 3.15
Longitudinal view of the palmar aspect of the proximal interphalangeal joint showing B-mode flexor tenosynovitis (asterix) (a) with Doppler signal (b); ft flexor tendon, pp proximal phalanx, dp distal phalanx
The detection of erosions is also useful in RA diagnosis. Although erosions can be detected in several rheumatic diseases, some areas can be considered as target for RA. US assessment of the styloid process of the ulnar, the radial part of second MCP joint, and the ulnar part of fifth MCP joint can provide important information for RA diagnosis. Zayat et al. investigated the specificity and sensitivity of US-detected erosion in RA compared to different musculoskeletal diseases (i.e., psoriatic arthritis (PsA) , osteoarthritis (OA), and gout) and healthy controls. Although RA patients presented more US-detected erosions than other groups, the differences were not significant. When RA-target sites only were included, i.e., second and third MCP, fifth MTP, and distal ulna, the sensitivity improved but was still not specific for RA. However, the presence of large erosions covering between one- to two-thirds of the surface of one quadrant in any of RA-target sites was highly specific for RA. Furthermore, the presence of any erosion at the level of fifth MTP was both specific and sensitive for RA [90].
New classification criteria for RA have been developed by American College of Rheumatology (ACR) and EULAR and published in 2010. A number of recent studies have shown the value of adding US in making the diagnosis of RA [82, 91]. Furthermore, based on US finding, patients were more accurately classified as requiring MTX treatment [82].
In addition to its diagnostic role, the US-detected inflammation can also be used to predict the progression of undifferentiated inflammatory arthritis to RA. Salaffi et al. found that the strongest independent predictor factor for developing RA in early, undifferentiated arthritis was PD positivity. Moreover, the positivity of PD in more than three joints increased significantly the risk of progression to RA [92]. Furthermore, van de Stadt et al. found that the presence of both BM and PD synovitis increases the risk for the development of arthritis in patients with arthralgia, without arthritis at clinical examination and positive anti-citrullinated protein antibodies (ACPA) and/or immunoglobulin M-rheumatoid factor (IgM-RF) [93]. In another work, Navalho et al. studied the association between MRI-detected synovitis and tenosynovitis at hand level with progression to RA. They found that ECU tenosynovitis, finger flexor tenosynovitis of the second finger, and radio-carpal synovitis were significantly associated with progression to RA [94].
In other rheumatic diseases, joint inflammatory activity can also be detected. Synovitis, tenosynovitis, erosions, and Doppler signals were reported in a variety of inflammatory and noninflammatory diseases, e.g., PsA, OA. Some US findings can help differentiate RA from other inflammatory diseases. For example, peritenon inflammation of finger extensor tendons is highly characteristic of PsA [95], while Doppler activity at the enthesis level is characteristic in SpA patients [96].
However, it should be highlighted that RA patients may also experience other rheumatic diseases that have different treatments and prognosis. Joints included in clinical scores (e.g., shoulder, knee) are often affected by degenerative processes and clinical differentiation of these pathologies can be challenging. Figure 3.16 shows an example of degenerative changes of the knee joint in an RA patient with knee pain, whereas, Fig. 3.17 shows an example of full-thickness tear of the supraspinatus tendon in an RA patient with shoulder pain. MSUS is useful in identifying pathologic changes related to degenerative musculoskeletal disorders (e.g., OA) or regional pain syndromes in RA patients, thus helping in differentiating these pathologies from active disease. Figure 3.18 shows an example of BM synovitis with Doppler signal of a PIP joint in an OA patient.
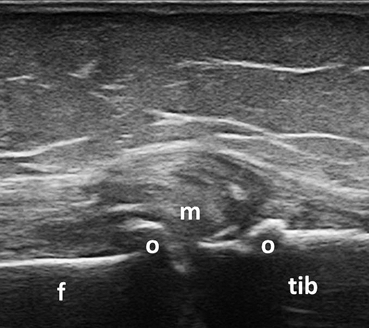
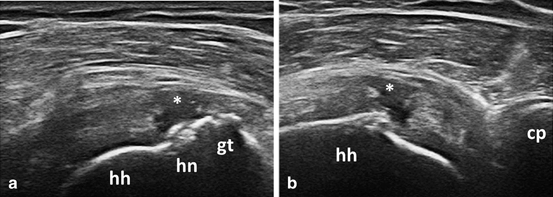
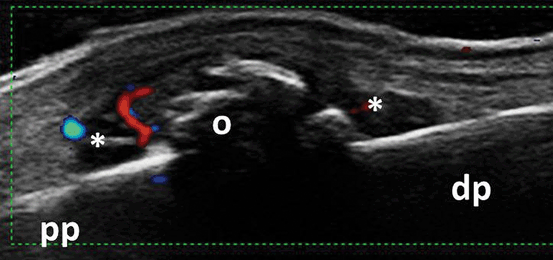

Fig. 3.16
Longitudinal view of the medial aspect of the knee joint showing degenerative changes in a patient with rheumatoid arthritis and concomitant osteoarthritis; f medial femoral condyle, tib tibia, m medial meniscus, o osteophytes

Fig. 3.17
Longitudinal (a) and transversal (b) view over the supraspinatus tendon showing fullthickness tear (asterix); gt greater tuberosity, hn anatomic humeral neck, hh humeral head, cp coracoid process

Fig. 3.18
Longitudinal view of the dorsal aspect of the proximal interphalangeal joint showing B-mode synovitis (asterix) with Doppler signal and osteophytes in a patient with osteoarthritis; pp proximal phalanx, dp distal phalanx, o osteophytes
Furthermore, in patients already diagnosed with RA, MSUS can help to differentiate active disease from chronic structural damage. Although there is no consensus in the definition of US-active synovitis, the presence of Doppler signal is considered as a sign of active inflammation. At tendon level, MSUS can help in differentiating active tendon inflammation from chronic tendon damage. Although clinical examination can identify complete tendon tear, partial tear remains undiagnosed. Thus, tendon damage although secondary to persistent tendon inflammation, does not represent active disease; thereby does not require changes in DMARDs treatment.
Monitoring Disease Activity
In several studies, MSUS has shown a good sensitivity to change [15, 42–48]. When compared to MRI, MSUS showed a high sensitivity in detecting both synovitis and tenosynovitis [21, 59, 64]. MSUS was also reported to be more sensitive than CR and as sensitive as MRI, in detecting bone erosion [69]. In addition, earlier studies revealed that MSUS was more sensitive than clinical assessment in detecting joint inflammation [31, 47, 79]. All the evidence coming from the studies points to MSUS as a useful and valuable tool in monitoring RA patients. Taking all of this into consideration, EULAR recommendations for the use of imaging in RA endorsed the use of US for more accurate assessment of inflammation [23].
As far as monitoring disease activity, a comprehensive US assessment of all accessible peripheral joints would be time-consuming and not necessarily practical in daily clinical practice. However, until now, there is no consensus on how many and which joints should be assessed. Several reduced joint counts that have been studied are discussed below. Scheel et al. suggested that US evaluation of second to fourth PIP and MCP joints with a semiquantitative score is sufficient for diagnosis and follow-up in RA patients [33]. A study carried out by Naredo et al. depicted high correlation of a reduced 12-joint count, i.e., bilateral elbow, wrist, second and third MCP and PIP, knee and ankle, with a comprehensive 44-joint count. Moreover, this reduced 12-joint count was shown to represent accurately the response to biologic therapy in RA patients [46]. In another study, Backhaus et al. developed a reduced US joint count which included assessment of only seven small joints, i.e., wrist, second and third MCP and PIP, second and fifth MTP of the clinically dominant side, combining soft tissue changes such as synovitis, tenosynovitis, paratenonitis with erosive bone lesions. This US seven-joint count showed to be a sensitive tool in monitoring patients with inflammatory arthritis (i.e., RA and PsA) in daily clinical practice [97]. This score was found to be sensitive to change in a cohort of patients with RA treated with csDMARDs or bDMARDs [98]. According to the study by Perricone et al., a reduced 6-joint count, i.e., bilateral wrist, second MCP, and knee, correlated excellently with the 12-joint count and was also shown to be sensitive to change in RA patients treated with etanercept (ETA) [99].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree