Chapter 74 Andrada R. Popescu, Darshit Thakrar, Stanley T. Kim, Emma E. Boylan, R. Andrew Defreitas and Cynthia K. Rigsby Overview: Ebstein anomaly accounts for fewer than 1% of all cases of congenital heart disease.1 Associated malformations include ventricular septal defect (VSD), pulmonary stenosis and atresia, tetralogy of Fallot, congenitally corrected transposition of the great arteries, and patent ductus arteriosus (PDA) or atrial septal defect (ASD).1 Conduction system abnormalities and arrhythmias, including Wolff-Parkinson-White syndrome and right bundle branch block, have been seen in 22% to 42% of patients.2 Etiology, Pathophysiology, and Clinical Presentation: The Ebstein anomaly is named for Wilhelm Ebstein, who described the abnormality in 1866.3 The anomaly is characterized by abnormal development and positioning of the tricuspid valve leaflets, with apical displacement of the septal and posterior valve leaflets into the right ventricle (RV). This phenomenon leads to partitioning of the RV into an atrialized basal segment and an apical outflow chamber. The displaced valve leaflets may be either adherent or nonadherent to the right ventricular wall. The anterior valve leaflet tends to be normally positioned, is frequently large and redundant, and sometimes is adherent to the right ventricular free wall with a “sail-like” appearance.4 The pathophysiology of this lesion varies, depending on the degree of tricuspid valve dysplasia and displacement of the tricuspid valve leaflets and the presence and severity of associated defects. With greater degrees of dysplasia and displacement, severe tricuspid insufficiency is increasingly present, resulting in elevated right atrial pressures and thus right-to-left shunting through either the ASD or the patent foramen ovale (PFO). Patients with mild forms of Ebstein anomaly may be asymptomatic in childhood and present with symptoms of cyanosis, right heart failure, or arrhythmia in adulthood. Patients with severe forms present in early neonatal life with severe combined cyanosis and acidosis and a duct-dependent circulation.5 Imaging: The chest radiographic appearance depends on the degree of tricuspid valve dysplasia and displacement. Classically, the chest radiographic appearance includes a globular or box-shaped heart as a result of right atrial enlargement associated with normal to diminished pulmonary vascularity (Fig. 74-1).6 Patients with significant valvular dysplasia and severe tricuspid insufficiency have marked enlargement of the right heart and decreased pulmonary blood flow. With less marked valve displacement and tricuspid insufficiency, only mild right heart enlargement may be present (e-Fig. 74-2). Figure 74-1 The classic radiographic appearance of Ebstein anomaly. e-Figure 74-2 Ebstein anomaly. Ebstein anomaly can be readily diagnosed by both prenatal and postnatal echocardiography.5 Cardiac magnetic resonance imaging (MRI) has emerged as a more complete method to evaluate the RV both anatomically and functionally.7,8 The characteristic features include displacement and tethering of the septal and posterior leaflets of the tricuspid valve and enlargement of the right atrium, including the atrialized RV (Fig. 74-3). Imaging goals include evaluation of the appearance of the tricuspid valve and the severity of tricuspid regurgitation; determination of the degree of right-to-left shunting through the ASD or PFO, which typically is present; evaluation of the volume of the atrialized and functional portions of the RV and RV systolic function; imaging for areas of fibrosis in the thin atrialized ventricular wall and septum on delayed gadolinium enhancement imaging9; assessment of the pulmonary valve and branch pulmonary arteries for stenosis; evaluation of left ventricle size and systolic function; and assessment of the heart and great vessels for other congenital abnormalities.2,7–9 Figure 74-3 Ebstein anomaly magnetic resonance image (MRI). Treatment: Patients with mild forms of Ebstein anomaly may not require surgical treatment, or the need for surgery may not arise until later in life if right heart dysfunction develops. Indications for surgical intervention/reintervention and/or medical therapy include limited exercise capacity (greater than New York Heart Association class II), increasing heart size (cardiothoracic ratio >65%), cyanosis (resting oxygen saturations of <90%), severe tricuspid regurgitation with symptoms, transient ischemic attack, or stroke.10 If surgery is required, valvuloplasty with either the Carpentier or cone procedures may be performed. The Carpentier procedure uses reimplantation of the anterior and posterior tricuspid valve leaflets at the level of the neotricuspid annulus, resulting in a monocuspid or bicuspid valve configuration.11 In the more recently developed cone procedure, the tricuspid valve leaflets and subvalvar apparatus are mobilized and reanastomosed to form a cone-shaped valve with improved inflow and valve competence.4 Other possible treatments include tricuspid valve replacement or, rarely, oversewing of the tricuspid valve and creation of a central shunt in infancy. Transcatheter closure of the ASD may be considered if severe cyanosis is present without tricuspid insufficiency necessitating valve repair.12 In rare situations, cardiac transplantation may be indicated.5 Clinically significant arrhythmias develop in some patients and require transcatheter or surgical ablation procedures.2 Overview: Tricuspid atresia features an absence of the tricuspid valve with a resultant lack of a direct connection between the right atrium and RV. Tricuspid atresia accounts for approximately 3% to 4% of congenital heart disease.13 Most cases of tricuspid atresia are sporadic, but familial cases and association with 22q11 microdeletions have been reported.14 Pathophysiology and Clinical Presentation: The tricuspid valve is usually absent; instead, muscular tissue or, less commonly, fibrous tissue is present in the floor of the right atrium. Because of the absence of a communication between the right atrium and the RV, an obligatory ASD or PFO is present to allow blood returning to the right side of the heart to reach the left side of the heart. This phenomenon results in mixing of systemic venous and pulmonary venous return and a variable degree of cyanosis. A VSD also may be present, with the degree of RV development related to the size of the VSD.13 In 70% of cases, the ventriculoarterial connections are concordant, and usually severe pulmonary or subpulmonary stenosis is present. In 30% of cases, the ventriculoarterial connections are discordant (transposition of the great arteries), and associated mild pulmonary stenosis and obstruction to aortic outflow is present. The most commonly used classification system was developed by Tandon and Edwards in 1974; they describe tricuspid atresia as types I, II, and III (Table 74-1).15 The clinical presentation is variable. The severity of cyanosis is related to the degree of RV outflow obstruction, the size of the VSD, the origins of the great arteries, the presence or absence of a PDA, and the pulmonary vascular resistance. All these factors will determine the relative blood flow to the pulmonary versus the systemic vasculature. If an infant has a large VSD and no obstruction to pulmonary outflow or a large PDA, the relative flow to the pulmonary circulation is much greater than that to the systemic circulation. This phenomenon may result in mild cyanosis with overcirculated, congested lungs. In contrast, restricted pulmonary blood flow results in more severe cyanosis.13 Imaging: The appearance of the heart on a chest radiograph depends on the specific anatomic type of tricuspid atresia; the heart usually appears normal or may be mildly enlarged. When pulmonary outflow obstruction is present, the chest radiograph shows normal to decreased pulmonary vascularity. When no pulmonary outflow obstruction is present, cardiomegaly and increased pulmonary vascularity usually are present (e-Fig. 74-4). e-Figure 74-4 Tricuspid atresia in a 4-month-old boy. The characteristic imaging feature of tricuspid atresia is replacement of the tricuspid valve with a ridge of muscular and fatty tissue positioned between the enlarged right atrium and the hypoplastic RV (Fig. 74-5). The size of the coexistent VSD and the relationship of the pulmonary artery and aorta can be characterized to determine the specific type of tricuspid atresia present. Echocardiography generally is the imaging study of choice in infancy, but MRI or computed tomography (CT) can be used to determine cardiac or great vessel anatomy in complex cases. Although cardiac catheterization traditionally has been performed before the second and third stages of univentricular repair, MRI is playing an increasing role in preoperative evaluation and may obviate the need for catheterization in carefully selected patients (see Treatment section).16 Important components of cross-sectional imaging before Glenn or Fontan procedures include assessment of anatomy and flow through the cavopulmonary pathway, atrioventricular valve function, and quantification of ventricular size and systolic function. MRI also allows detailed anatomic and functional assessment of the full Fontan pathway unless it is limited by artifact from implanted ferromagnetic material or pacemakers. MRI therefore is a valuable tool in the assessment of a “failing Fontan.” Figure 74-5 A 27-year-old with tricuspid atresia after extracardiac Fontan palliation (asterisk). Treatment: Ultimately, patients with tricuspid atresia require univentricular palliative repair because the hypoplastic RV is not capable of providing the cardiac output necessary to support a two-ventricle heart. Neonates with tricuspid atresia and associated pulmonary atresia or stenosis may require a prostaglandin infusion to keep the PDA open until surgical repair is performed. In an infant with a large VSD and no pulmonary stenosis, diuretics may be needed to decrease pulmonary overcirculation. The goal of univentricular repair is to eliminate cyanosis.17 This is accomplished by having the single left ventricle support the systemic circulation and by directing the systemic venous return to the pulmonary arteries, thus bypassing the nonfunctional RV. The first stage of single ventricle palliation performed in early infancy ensures adequate but not excessive pulmonary blood flow. Infants with pulmonary stenosis or atresia may require a modified Blalock-Taussig systemic to pulmonary artery shunt to maintain adequate pulmonary flow as the first stage of univentricular repair (see also Chapter 75). Alternatively, pulmonary arterial banding may be performed in infants with excessive pulmonary blood flow because of a large VSD and no pulmonary hypoplasia or stenosis.
Right Heart Lesions
Ebstein Anomaly
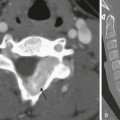
A frontal view of the chest shows globular box-shaped heart enlargement and diminished pulmonary vascularity.
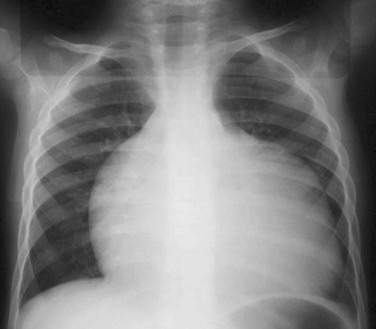
A frontal chest radiograph in a mildly cyanotic 7-day-old girl reveals mild to moderate enlargement of the heart and normal to decreased pulmonary vascularity as a result of a mild Ebstein anomaly.
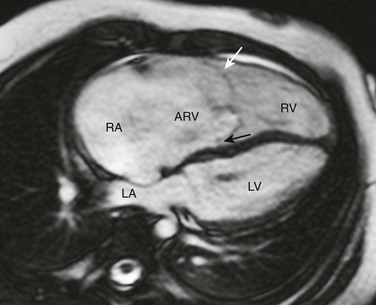
Axial steady-state free-precession MRI demonstrates right heart enlargement with redundancy of the anterior tricuspid valve leaflet (white arrow) and displacement of the septal tricuspid valve leaflet (black arrow) into the right ventricle (RV). As a result, a large atrialized portion of the right ventricle (ARV) is present. LA, Left atrium; LV, left ventricle; RA, right atrium.
Tricuspid Atresia
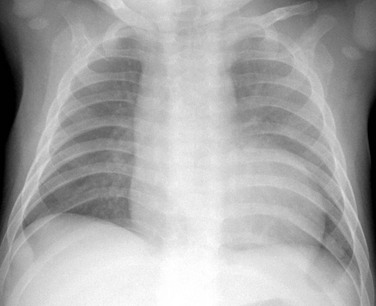
A frontal chest radiograph shows mild cardiac enlargement with a rounded apex and a concave left upper heart margin as a result of pulmonary atresia.
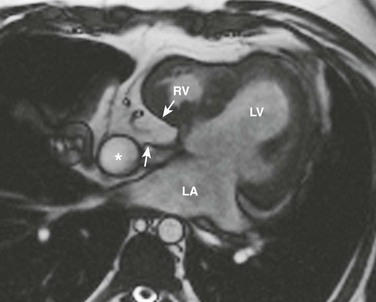
An axial magnetic resonance image of the heart shows a ridge of fatty tissue (arrows) in the floor of the right atrium at the site of the atretic tricuspid valve. A small caliber right ventricle (RV) also is present. LA, Left atrium; LV, left ventricle.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree