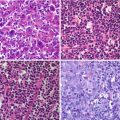
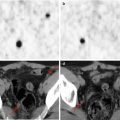
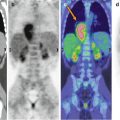
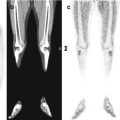
Fig. 19.1
Recurrent ganglioglioma in an 8-year-old girl. (a) Sagittal Gd-enhanced T1-weighted image; (b) 18F-DOPA–PET/MRI fusion image. There is a focal, rounded, contrast-enhancing lesion in the inferior frontal gyrus (thin arrow in a) with elevated 18F-DOPA activity (thin arrow in b). Normal tracer uptake in the adjacent striatum (open arrow in b) is seen as well
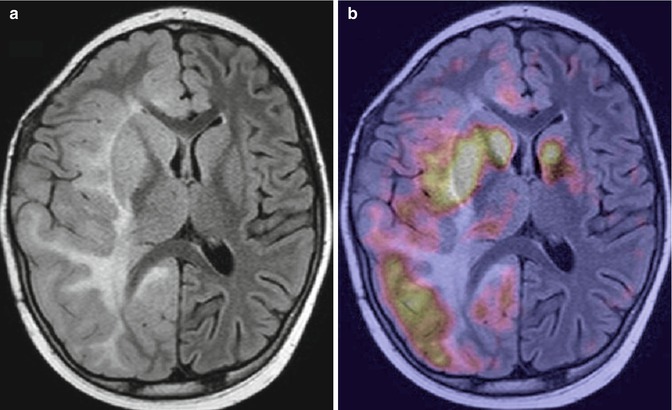
Fig. 19.2
Gliomatosis cerebri in a 10-year-old boy. (a) Axial fluid attenuated inversion recovery (FLAIR) image; (b) 18F-DOPA–PET/MRI fusion image. There is an extensive diffusely infiltrating lesion involving the right cerebral hemisphere (a) with heterogeneous, increased tracer uptake (b)
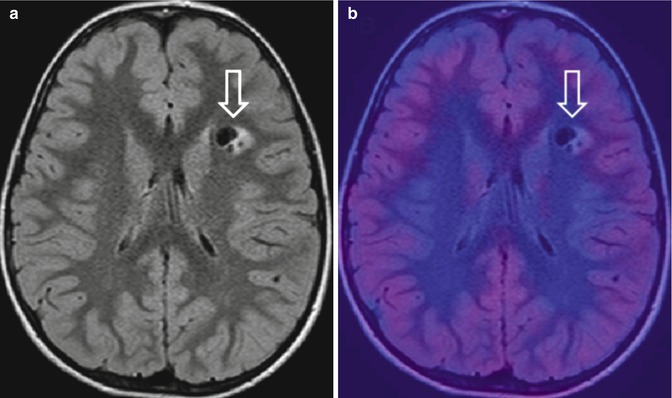
Fig. 19.3
Suspected brain tumor on MRI in a 12-year-old boy. (a) Axial FLAIR image shows a focal lesion within the left frontal white matter (open arrow) suspicious for a brain tumor. (b) 18F-DOPA–PET/MRI fusion image shows a lack of tracer uptake (open arrow). No neoplastic tissue was demonstrated after stereotactic biopsy
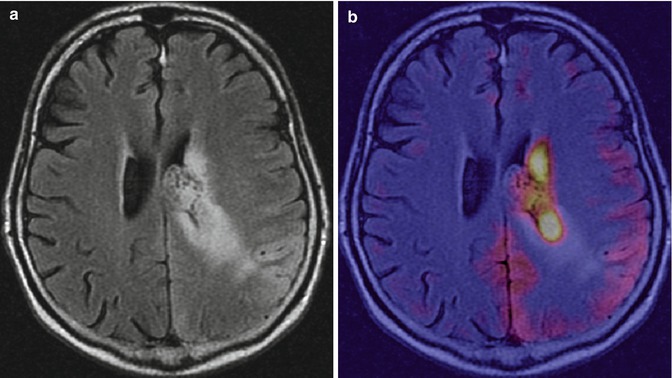
Fig. 19.4
Anaplastic astrocytoma in a 17-year-old boy. (a) Axial FLAIR image shows a periventricular expansive/infiltrating lesion with involvement of the left parietal cortex. (b) 18F-DOPA–PET/MRI fusion image shows heterogeneous, increased traced uptake
We recently studied a pediatric patient with malignant transformation of ganglioglioma treated with bevacizumab and were able demonstrate the significant contribution of combined multimodal MRI and 18F-DOPA–PET in the early diagnosis of tumor “pseudoresponse” and non-enhancing tumor progression [29]. Anti-angiogenic agents, especially those targeting vascular endothelial growth factor, such as bevacizumab, are increasingly used in pediatric patients with high-grade tumors. Previous studies in adults demonstrated that the differentiation between brain tumor response and tumor progression following treatment with bevacizumab poses a complex diagnostic challenge. This monoclonal antibody produces a dramatic decrease in the contrast enhancement of the lesion on conventional MRI, a phenomenon called “pseudoresponse.” The prefix “pseudo” refers to the fact that the imaging change reflects restoration of the blood–brain barrier rather than a true tumor response. Despite this stably reduced or absent contrast enhancement, patients can develop neurological worsening, with an increase of T2/FLAIR MRI abnormalities on follow-up studies, in keeping with a pattern of non-enhancing but progressive disease. Since an objective criterion for non-enhancing tumor progression is currently unfeasible, these patients must be closely and repeatedly followed until a confident disease evaluation is possible, as this will significantly influence decision-making regarding treatment continuation or discontinuation.
Our results suggest that 18F-DOPA–PET imaging in patients treated with anti-angiogenesis agents deserves further investigation to evaluate the potentially promising role of this novel imaging modality [29]. Overall, on the basis of our preliminary experience in pediatric patients with brain tumors, the results obtained with18F-DOPA confirm previous data from adults [30] in terms of the impact of this tracer on patient management and/or treatment decisions. Further investigations with larger series are thus also needed to validate the use of amino acid PET tracers in improving diagnostic accuracy, monitoring dynamic changes within brain tumors during therapy, and establishing whether (and if so, how and when) these radiolabeled amino acids should become a critical part of the clinical management of pediatric patients with brain tumors. Correlation with MRI data is mandatory for an accurate interpretation of PET results and thus a close collaboration between neuroradiologists and nuclear medicine physicians. Greater efforts within the diagnostic imaging community should be directed at improving the integration of information obtained by different imaging modalities in order to overcome their respective limitations. The result will be a more robust tool in the crucial evaluation of pediatric brain tumors.
Teaching Point
Amino acid PET tracers have an important role in adult brain tumors, influencing treatment management. The overall diagnostic accuracy of these tracers for brain tumor evaluation is higher than that of 18F-FDG [17]. Tumor detection and grading, biopsy, radiotherapy planning, posttreatment monitoring, and the evaluation of recurrent disease are among the most important applications [18]. Although few data are available on the role and impact of these newly developed tracers in pediatric brain tumors, 11C-methionine and 18F-DOPA have thus far shown great promise based on their ability to add useful diagnostic information to clinical and conventional MRI data. To further increase their diagnostic role, a correlation between the results obtained with these tracers and MRI findings is mandatory.
References
1.
Muller S, Chang S (2009) Pediatric brain tumors: current treatment strategies and future therapeutics approaches. Neurotherapeutics 6:570–586CrossRef
2.
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35:1773–1782PubMedCrossRef
3.
Pötter R, Czech TH, Dieckmann I et al (1998) Tumors of the central nervous system. In: Voute PA, Kalifa C, Barrett A (eds) Cancer in children. Clinical management, 4th edn. Oxford University Press, New York, pp 170–193
4.
Panigrahy A, Blüml S (2009) Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol 24:1343–1365PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree