and Laurence Loeuillet2
(1)
Centre d’échographie Ambroise Paré, Les Mureaux, France
(2)
Hôpital Cochin, Paris, France
Skull Contour Anomalies
Acrania
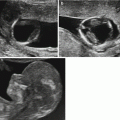
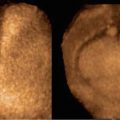
As we have already seen, acrania is generally diagnosed in the first trimester, but should no ultrasound examination have been performed in the first trimester, the sonographer may be faced with the classic image of the “reptilian” face (Fig. 7.1).


Fig. 7.1
Anencephaly
Opacities in Skull Images
Meningocele and Meningoencephalocele
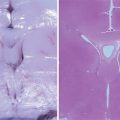
The meningoencephalocele is made up of meninges and brain tissue that yield images of mixed echogenic structure. The underlying brain structures are disorganized.
It should be noted that the size of the meningoencephalocele is not necessarily related to the extent of the brain lesions (Fig. 7.3a) (Kesrouani 2010).




Fig. 7.3
(a) Meningoencephalocele at 16 gw (triplane image and surface rendering). (b) Meningoencephalocele: fetal pathology specimens at 20 gw. (c) Small meningoencephalocele accompanying major hydrocephalus. (d) Meningoencephalocele: fetal pathology specimens. (e) Meningoencephalocele and cystic dysplasia at 22 gw in connection with Meckel-Gruber syndrome. (f) Meckel-Gruber syndrome: fetal pathology specimens: a encephalocele, b hexadactyly, c cystic renal dysplasia
Hemangioma of the Scalp
Skull Contour Anomalies Related to Neural Tube Closure Anomalies
Open spina bifida, and particularly that with myelomeningocele, is accompanied by cerebral signs: the frontal bones of the skull are deformed by inward scalloping, giving the classic “lemon” sign reported by K. Nicolaides et al. (1986). This deformation of the frontal bones may be more or less pronounced (Fig. 7.5a, b).


Fig. 7.5
(a, b) Deformation of the cranium related to neural tube closure anomalies. (Yellow arrows) deformation of frontal bones (convex to interior)
The fact that no cerebral signs are present should not lead the sonographer to rule out neural tube closure anomalies as these signs are absent in some 10 % of cases, particularly in cases of closed spina bifida or spina bifida with lipoma (Ghi et al. 2006).
Skull Contour Anomalies Related to Aneuploidy
In a number of cases, chromosomal anomalies are accompanied by particular skull shapes: bracycephaly in cases of trisomy 21 (Fig. 7.6a) and, “strawberry” sign in cases of trisomy 18 (Fig. 7.6b), mentioned here simply as a reminder. These cases of aneuploidy can be detected by careful screening in the first trimester and detailed morphological examinations conducted early.


Fig. 7.6
(a) Bracycephaly in trisomy 21, (b) “strawberry” sign in trisomy 18
Craniosynostoses
The premature fusion of one or several sutures of the skull results in deformation of the fetal cranium. A classification of these deformations may be established depending on the sutures seen to fuse prematurely (Fig. 7.7).


Fig. 7.7
Classification of cranial deformations in cases of craniosynostosis
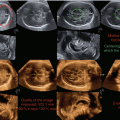
Conventional ultrasound provides only a limited visualization of the cranial sutures and fontanels. 3D ultrasound provides an “in front” view (Fig. 7.8) (Dikkeboom et al. 2004) of these structures and thereby provides for a more precise diagnosis.


Fig. 7.8
3D ultrasound with “in front” view technique
The example below shows premature fusion of the sagittal suture at 24 gw causing scaphocephaly and clearly illustrates the advantages of 3D ultrasound (Fig. 7.9a, b).


Fig. 7.9
(a) Scaphocephaly. (b) (Left) premature fusion of the sagittal suture at 24 gw, (right) normal appearance at the same term
Other Examples of Skull Deformations Related to Craniosynostosis

Fig. 7.10
(a) “Cloverleaf” skull (left) caused by fusion of the coronal sutures, (right) images of the interior of the skull showing imprints of bony “fingers”. (b) “Cloverleaf” skull: a fetal pathology specimen, b X-ray image. (c) “Cloverleaf” skull: fetal pathology specimen, (c) bony “fingers” imprint inside the skull

Fig. 7.11
(a) left, tower skull (fusion of the coronal sutures), right, trigonocephaly (fusion of the metopic suture). (b) craniosynostosis at 30 gw, fetal pathology specimens: fusion of the coronal sutures and protrusion of the metopic suture
It should also be noted that craniosynostosis may initially be detected as a compensatory widening of one or several other sutures.
The discovery of such a skull contour anomaly caused by craniosynostosis should prompt a full examination and in particular genetic testing (Delahaye et al. 2003).
Midline Anomalies
Holoprosencephaly
Alobar holoprosencephaly should have been diagnosed in the first trimester. However, and in particular if no ultrasound scan was performed in the first trimester, the diagnosis may only be suggested during the second-trimester morphological examination.
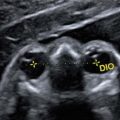
The sonographic signs are of course identical to those described for the first trimester, particularly a single ventricle, hypotelorism or cyclopia, or a proboscis; the nose may be absent or reduced to a single nostril. 3D ultrasound in maximum mode may show fusion of the frontal bones.


Fig. 7.12
(a) Alobar holoprosencephaly in the second trimester, a single ventricle, b single nostril, c proboscis, d fusion of the frontal bone plates. (b) Alobar holoprosencephaly at 22 gw: fetal pathology specimens, a dorsal vein in situ, b posterior coronal image, c single nostril and premaxillary agenesis
Semilobar holoprosencephaly: the fusion in general involves only the anterior part of the brain causing less marked fusion of the ventricles than in the alobar form. The thalami are only partly merged; the posterior part of the midline is present; the corpus callosum may in part be present (mainly at the level of the splenium) (Fig. 7.13a, b).


Fig. 7.13
(a) Semilobar holoprosencephaly at 23 gw (the posterior fossa here is abnormal). (b) Semilobar holoprosencephaly: fetal pathology specimens
Lobar holoprosencephaly (Fig. 7.14) is the most difficult to diagnose: fusion concerns only a small part of the brain: the corpus callosum is present and often dysmorphic. A rupture may be seen in the continuity between the frontal horns of the lateral ventricles. Sometimes, only the frontal lobes are merged. In such cases, it is particularly interesting to visualize the paths of the anterior cerebral arteries for if the frontal hemispheres are merged, the cerebral arteries cannot follow their usual pathways. They pass beneath and in front of the merged frontal lobes and run beneath the table of the bone, giving the appearance of the “cerebral artery crawling under the skull” (Fig. 7.15) (Bernard et al. 2002; Blin et al. 2004).



Fig. 7.14
Lobar holoprosencephaly at 23 gw, (a) minimal fusion of the anterior part of the lateral ventricles, (b) 3D “reverse face” view

Fig. 7.15
“Cerebral artery crawling under the skull” at 22 gw: the green arrows indicate the path of the anterior cerebral arteries that run beneath the frontal bones. Right control image: normal path of the cerebral arteries
Corpus Callosum Anomalies
Agenesis of the Corpus Callosum
This may be complete or partial.
Complete Agenesis
In most cases, the diagnosis is made on the basis of indirect signs:









In a coronal image: the frontal horns of the lateral ventricles show the characteristic feature of being indented, giving the characteristic so-called bulls horn” appearance (Fig. 7.17a, b).

Fig. 7.17
(a) Complete corpus callosum agenesis: indented frontal horns. (b) Complete corpus callosum agenesis at 35 gw, fetal pathology specimens: frontal horns drawn out to form “bull’s horns” and cavum septum pellucidum absent

Fig. 7.18
Complete corpus callosum agenesis: “tear drop” configuration of the posterior horn


Fig. 7.20
(a) Complete corpus callosum agenesis. Radial folds (third trimester). (b) Complete corpus callosum agenesis at 32 gw: fetal pathology specimens: radial folds
Cavum septum pellucidum absent and ascent of V3: absent corpus callosum accompanied by absent cavum septum pellucidum allowing ascent of the V3 (Fig. 7.21a). Triplane 3D ultrasound can be used to clearly visualize this anomaly in all three planes (Fig. 7.21b) (Pilu et al. 2006, 2007; Plasencia et al. 2007).

Fig. 7.21
(a) Anterior part of the ascending V3 (yellow arrow). (b) Complete corpus callosum agenesis, triplane ultrasound images: ascending V3 and cavum septum pellucidum absent

Fig. 7.22
Complete corpus callosum agenesis: ascending V3 (green outline)

Fig. 7.23
Complete corpus callosum agenesis: false cavum septum pellucidum

Fig. 7.24
Complete corpus callosum agenesis: abnormal vascularization by TUI

Fig. 7.25
(a) Complete corpus callosum agenesis: abnormal vascularization (glass body mode). (b) Complete corpus callosum agenesis and abnormal vascularization (fetal pathology specimens), a at 36 gw, b corpus callosum and normal vascularization at 32 gw
Direct signs are visible on the sagittal image: the structure corresponding to the corpus callosum cannot be found (Figs. 7.26 and 7.27).



Fig. 7.26
Complete corpus callosum agenesis in fetus A, normal corpus callosum in fetus JB (Dichorionic-diamniotic twin pregnancy at 22 gw)

Fig. 7.27
Complete corpus callosum agenesis at 32 gw
Partial Agenesis
Given the development of the corpus callosum (see Chap. 3 on the normal corpus callosum), this generally concerns the most posterior part of the corpus callosum (Fig. 7.28) (the splenium). But partial agenesis of the anterior part may also be encountered (Fig. 7.29). Partial agenesis is clearly visible in the strict sagittal image.
Indirect signs are often seen, in particular indenting of the frontal horns and above all colpocephaly (Volpe et al. 2006).



Fig. 7.28
(a) Partial agenesis of the posterior part of the corpus callosum. (b) Agenesis of the posterior part of the corpus callosum at 34 gw (fetal pathology specimens)

Fig. 7.29
Partial agenesis of the anterior part of the corpus callosum. (Yellow arrows) deformation of frontal bones (convex to interior)
Short, Thick Corpus Callosum
In a number of cases, the corpus callosum may appear to be short and thick (see Chap. 8 for length and thickness of the corpus callosum) (Fig. 7.30).


Fig. 7.30
(a) Short, thick corpus callosum. (b) Short, thick corpus callosum at 35 gw (fetal pathology specimens)
Should complete or partial agenesis be detected or the corpus callosum have a dysmorphic appearance, a full examination is required combining tests for genetic anomalies with a full morphological examination (Volpe et al. 2006; Ghi et al. 2010; Lerman-Sagie et al. 2009; Fong et al. 2004; Godard et al. 2005), with a special focus on detecting associated cerebral signs such as those illustrated in the two examples below (Figs. 7.31 and 7.32).



Fig. 7.31
Short, dysmorphic corpus callosum at 24 gw in connection with Miller-Dieker lissencephaly

Fig. 7.32
Dysmorphic corpus callosum and facial dysmorphy in trisomy 8
Lipomas of the corpus callosum will be addressed in the section on intracranial expansive processes.
Anomalies of the Septum Pellucidum
Absence of the cavum septum pellucidum has already been addressed in the section on corpus callosum agenesis. Here in this section, we will address the question of septal agenesis.
Septal Agenesis
It is generally in the coronal section passing through the interface between the anterior third and the posterior two-thirds of the brain that this type of anomaly can best be visualized. It is characterized by nonvisualization of the lateral walls of the cavum septum pellucidum (Fig. 7.34a, b), giving a “butterfly wings” appearance caused by fusion with the frontal lobes of the lateral ventricles. The anomaly is also visible in the axial image. The corpus callosum is normal in appearance (Fig. 7.33).



Fig. 7.33
Septal agenesis: triplane images illustrating the level of the coronal section (white double arrow)

Fig. 7.34
(a) Septal agenesis coronal image: lateral walls of the septum absent. (b) Septal agenesis at 34 gw (fetal pathology specimens)
The diagnosis, with semilobar or lobar holoprosencephaly, is based on
Image shape: in holoprosencephaly the fusion of the septum with the anterior horns of the frontal horns appears to be far more extensive in coronal images,
The path taken by the anterior cerebral arteries, which, in septal agenesis, take the usual path unlike the “cerebral artery crawling under the skull” appearance in cases of holoprosencephaly (Fig. 7.15).
Discovery of septal agenesis should prompt further examinations aiming to rule out septo-optic dysplasia.
Although not constituting formal proof, if maternal estradiol levels are normal (Lepinard et al. 2005), if the posterior branches of the optic chiasma are normal in size by 3D ultrasound (Fig. 7.35a) (Bault 2006, 2007; Bault et al. 2011), and if the olfactory bulbs can be visualized (Fig. 7.35b), this would tend to rule out septo-optic dysplasia.


Fig. 7.35
(a) measurements of posterior branches of the optic chiasma (1 and 2), (b) olfactive bulbs
Special Features
The septum may on occasion be seen to have a particular appearance: the septum pellucidum may appear to be enlarged (Fig. 7.36) or echogenic (Fig. 7.37). The significance of these two signs is unknown, but they should prompt the sonographer to conduct a very careful examination of cerebral structures (Jou et al. 1998).



Fig. 7.36
Large septum pellucidum at 32 gw (median at this time point: 6.42 mm)

Fig. 7.37
Echogenic septum pellucidum
Ventriculomegaly
Definitions
A ventricle is considered to be too wide if atrial diameter throughout pregnancy, correctly measured (see the Chap. 3 on measurement of ventricles), exceeds 10 mm, which corresponds to a value above the 97th percentile (this value appears to be constant from 17 to 35 weeks of gestation) (Salomon et al. 2007).
Ventriculomegaly may be unilateral or bilateral for the lateral ventricles, triventricular if also involving the V3, or quadriventricular if including the V3 and the V4.
Ventriculomegaly is considered to be mild if between 10 and 12 mm, moderate if between 12 and 15 mm, and severe if greater than 15 mm (Fig. 7.38a, b, c).


Fig. 7.38
(a) Ventriculomegaly types: a mild, b moderate, c severe. (b) Ventriculomegaly in a fetus with trisomy 21 (low risk in the first trimester, brachycephaly). (1) width of lateral ventricle
Etiologies
The discovery of ventriculomegaly should prompt a full investigation for its etiology.
In this chapter, we illustrate the main ultrasound signs that can point to the etiology of ventriculomegaly.
Chromosomal Anomalies and Ventriculomegaly
Trisomy 21 is the chromosomal aberration with the highest prevalence of ventriculomegaly, even though this prevalence is fairly low (6 cases for 140 cases of trisomy 21) (Albig et al. 2006) (15/529 in a meta-analysis, i.e., an LHR of +9 as published by Melchiorre et al. in 2009), 3.8 % for Pilu et al. in 1999. The frequency of such ventriculomegaly, of course, depends on previous screening. Most cases of ventriculomegaly are mild (Fig. 7.38b).
Ventriculomegaly Related to an Anomaly of the Cerebral Aqueduct
Ventriculomegaly Due to Aqueduct Stenosis
Generally, the image produced is that of triventricular ventriculomegaly. It may be detected early, particularly in cases of dominant aqueduct stenosis (Robyr et al. 2005) (Fig. 7.39).
This appearance is characteristic of X-linked hydrocephalus: Bickers-Adams syndrome where triventricular hydrocephalus is associated with the presence of clasped thumbs. This hydrocephalus is caused by a mutation of the L1CAM gene.




Fig. 7.39
Triventricular ventriculomegaly by stenosis of the cerebral aqueduct at 15 gw

Fig. 7.40
Ventriculomegaly by mutation of the L1CAM gene in a male fetus: relapse at 16 gw

Fig. 7.41
(a) Ventriculomegaly in Bickers-Adams syndrome (28 gw), image shows clasped thumbs. (b) Bickers-Adams syndrome (fetal pathology specimens): a Triventricular hydrocephalus at 18 gw, b clasped thumbs at 22 gw. (c) Atresia (forking) of the cerebral aqueduct extending to the third ventricle at 32 gw, marked dilation of the lateral ventricles
The discovery of triventricular ventriculomegaly in a male fetus should prompt an investigation into family history, an ultrasound scan for clasped thumb, and, depending on the outcome of the genetic consultation, a test for mutation of the L1CAM gene (Figs. 7.39, 7.40, and 7.41).
Ventriculomegaly due to atresia of the aqueduct: this form of ventriculomegaly is related to a total absence of lumen in the aqueduct and in general stems from lesions acquired through infection or hemorrhage. It may be found in malformations and may sometimes be biventricular.
Ventriculomegaly in a Setting of Neural Tube Closure Anomalies
The discovery of ventriculomegaly should prompt investigations for other cerebral signs of neural tube closure anomalies: “lemon” sign by deformation of the frontal bone, “banana” sign cerebellum, and Arnold-Chiari malformation (Fig. 7.42). Obviously, special care must be taken when examining the spine for direct signs (Fig. 7.43) while bearing in mind that approximately 10 % of spina bifida cases do not show cerebral signs (Fig. 7.44) (Ghi et al. 2006).







Fig. 7.42
(a) a Ventriculomegaly in spina bifida, b “lemon” signs, c Arnold-Chiari malformation. (b) Open spina bifida (fetal pathology specimens): a macroscopic features, b ventricular dilation, c Arnold-Chiari malformation


Fig. 7.43
(a) Direct signs of spina bifida in the spinal cord. (b) Open spina bifida (fetal pathology specimens), upper spinal anomalies by X-ray, lower myelomeningocele with ulceration at 26 gw

Fig. 7.44
“Closed” spina bifida devoid of cerebral signs at 26 gw
Ventriculomegaly Accompanied by Other CNS Malformations
Agenesis of the Corpus Callosum
In agenesis of the corpus callosum, ventriculomegaly takes the form of colpocephaly (see section “Midline anomalies”). As indicated previously, investigations should be made for other indirect and direct signs of agenesis of the corpus callosum (Fig. 7.45).


Fig. 7.45
Ventriculomegaly in a case of complete agenesis of the corpus callosum (frontal horns scalloped outward)
Ventriculomegaly may also be seen in cases of partial agenesis of the corpus callosum (Fig. 7.46).


Fig. 7.46
Severe ventriculomegaly and partial agenesis of the corpus callosum
Agenesis of the Septum Pellucidum
Ventriculomegaly may be associated with absence of the septum pellucidum. Here, when making the diagnosis, it is essential to differentiate between agenesis of the septum pellucidum with its risk of septo-optic dysplasia and the various types of holoproscencephaly (see “Midline anomalies”) (Malinger et al. 2005).


Fig. 7.47
Ventriculomegaly with agenesis of the septum pellucidum
Arachnoid Cysts
Certain very large arachnoid cysts may cause compression of the cerebral aqueduct and give the appearance of triventricular ventriculomegaly (Fig. 7.48).


Fig. 7.48
Triventricular ventriculomegaly caused by compression by a large arachnoid cyst
Anomalies of the Posterior Fossa
A good number of pathologies of the posterior fossa are accompanied by ventriculomegaly (see chapter on pathologies of the posterior fossa).
In particular, we may cite Joubert’s syndrome (Fig. 7.49) and rhombencephalosynapsis (Fig. 7.50).



Fig. 7.49
Ventriculomegaly at 17 gw: Joubert’s syndrome (* dilated ventricles, arrows elongated superior cerebellar peduncles around the abnormally large V4)

Fig. 7.50
(a) Ventriculomegaly with rhombencephalosynapsis. (b) Fetal pathology features of rhombencephalosynapsis: cerebellum and ventriculomegaly
Anomalies of the Gyration
Gyration disorders such as type II lissencephaly are accompanied by ventriculomegaly (see the section “Gyration disorders”).


Fig. 7.51
(a) Ventriculomegaly at 24 gw. Type II lissencephaly: Walker-Warburg syndrome. (b) Ventriculomegaly and rupture of the septum pellucidum at 24 gw: Walker-Warburg syndrome (fetal pathology specimen)
Ventriculomegaly in Hemorrhagic or Ischemic-Hemorrhagic Pathologies
Examination of the ventricular walls and contents may indicate a pathology that is hemorrhagic or ischemic (often secondary to a hemorrhagic phase).
In cases of hemorrhage, the ventricular walls are echogenic, and the different echogenic intraventricular structures by their shape and the location of the choroid plexuses are suggestive of blood clots (Fig. 7.52).



Fig. 7.52
(a) Unilateral hemorrhagic ventriculomegaly at 23 gw (arrows blood clots). (b) Intra-ventricular, parenchymatous hemorrhage, ventricular dilation at 26 gw (fetal pathology specimen)

Fig. 7.53
Hemorrhagic ventriculomegaly at 32 gw (arrows blood clots)
Ischemic lesions may be detected in the form of irregular lesions of the ventricular walls which may appear to be “nibbled” (Fig. 7.54).


Fig. 7.54
Ventriculomegaly at 27 gw: Ischemic lesions secondary to hemorrhagic lesions
Infectious Ventriculomegaly
The appearance of the ultrasound images obtained in such cases will be addressed more in detail in the chapter devoted to infectious pathologies, but we may already note here that the outcome of infectious ventriculomegaly is very different depending on the type of pathogen: very rapid increase in toxoplasmosis against a background of microencephaly for cytomegalovirus (Fig. 7.55).


Fig. 7.55
Infectious ventriculomegaly: left CMV intraventricular septa and echogenic periventricular halo, right toxoplasmosis: calcification showing as occipital “candle wax drippings”
Ventriculomegaly in Cases of Brain Tumors
Such cases are generally asymmetrical and poorly codified. The cerebral parenchyma is pushed back or laminated by the tumoral tissue causing a “mass effect” (see the section “Intracranial expansive processes”) (Fig. 7.56).


Fig. 7.56
(a) Tumoral ventriculomegaly. (b) Embryonic tumor at 36 gw and secondary hydrocephalus (fetal pathology specimens)
The discovery of ventriculomegaly should prompt a full investigation that includes reference ultrasound scan, karyotyping, PCR for CMV and toxoplasmosis, fetal blood sampling (FBS) for CBC, and study of fetal hemostasis if signs are evident of hemorrhage, and genetic consultation in particular in cases of nonisolated ventriculomegaly; MRI will be performed mainly for ventriculomegaly exceeding 12 mm.
Ultrasound scans should be repeated every 2–4 weeks to monitor course.
Finally, it is noteworthy that ventriculomegaly generally has a good prognosis with 88 % of fetuses showing a favorable neurological outcome in the Melchiorre meta-analysis that studied ten series reported and published in 2009 (Melchiorre et al. 2009). The magnitude of the initial dilatation, the rate at which it increases, and the presence of associated signs are all factors that determine postnatal prognosis (Beeghly et al. 2010; Gaglioti et al. 2005).
Anomalies of the Posterior Fossa
Ever since 2006 and publication of Laurent Guibaud’s algorithm (Guibaud and des Portes 2006), the approach to anomalies of the posterior fossa has been far more clearly codified:
1.
The retrocerebellar cistern is increased:
The torcula is raised: Dandy-Walker anomaly
The torcula is in place: biometrics should be measured (transverse cerebellar diameter) and cerebral anatomy investigated
Cerebral anatomy is normal:
Mega cisterna magna
Arachnoid cyst
Cysts in Blake’s pouch
Cerebral biometrics are reduced (see § 2)
Cerebral anatomy is abnormal (see § 3)
2.
Cerebral biometrics are reduced:
(a)
a-anatomy normal:
Focal reduction
Overall reduction concerning the vermis and in certain cases the brainstem
(b)
b- cerebral anatomy abnormal: see § 3.
3.
Cerebral anatomy abnormal:
Rhombencephalosynapsis
Chiari II anomaly
Agenesis of the vermis
4.
Special forms:
Joubert’s syndrome and CHARGE syndrome are more difficult to classify since the retrocerebellar cisterna is increased, but this in fact is related to an abnormal small vermis.
Brainstem-vermis (BV) angle may also be measured.
Published normal values are 30° for BT angle and 10° for BV angle.


Fig. 7.57
Measuring angles in the posterior fossa at 25 gw: yellow BT angle, green BV angle
Thus, an open BT angle confirms that the cerebellar tentorium has been raised.
Also, a Doppler ultrasound scan could be used to locate the right sinus and therefore the cerebellar tentorium, particularly if large subtentorial arachnoid cysts are present (Fig. 7.58).