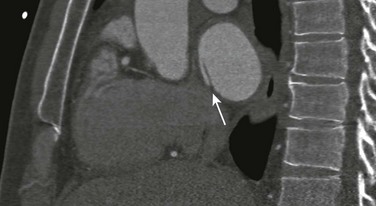
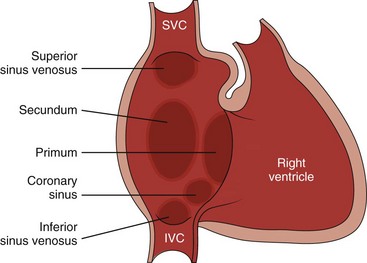
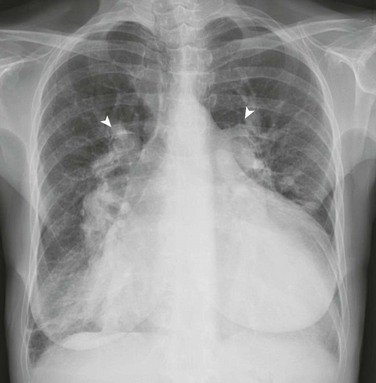
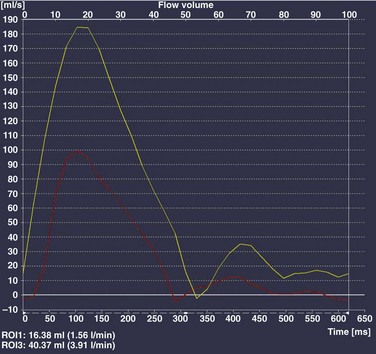
Chapter 73 Overview: An atrial septal defect (ASD) is a defect in the atrial septum that allows communication between the right and left atria. Isolated ASDs account for 6% to 10% of all cases of congenital heart disease. Etiology: Two primary types of ASDs occur and are classified by their relationship to the fossa ovalis (e-Fig. 73-1). Secundum defects (which comprise 80% to 90% of all ASDs) occur in the region of the fossa ovalis. Although a patent foramen ovale is in a similar location, it usually is not considered a septal defect but is the remnant of the normal interatrial communication present during fetal life and is present in 27% to 34% of the general population (Fig. 73-2).1,2 Ostium primum defects occur caudal to the fossa ovalis at the base of the atrial septum, are usually large defects, and almost always are associated with other types of structural heart disease. Additionally, two defects—a sinus venosus septal defect and an unroofed coronary sinus—do not involve the atrial septum but are physiologically equivalent to an ASD because they allow blood to shunt from the left atrium to the right atrium.3,4 Sinus venosus septal defects are located posterior to the fossa ovalis and occur as a result of a deficiency in the sinus venosus septum, which separates the right pulmonary veins from the superior vena cava and from the posterior aspect of the right atrium. This defect is usually located in the wall between the posterior and inferior border of the superior vena cava and the right atrium, and it is commonly associated with anomalous drainage of the right upper, middle, or lower pulmonary veins draining to either the right atrium or superior vena cava (see Chapter 72).5 An unroofed coronary sinus is rare and occurs as a result of a partial or complete absence of the wall between the inferior left atrium and the roof of the coronary sinus. An unroofed coronary sinus generally is associated with drainage of a left superior vena cava to the coronary sinus or left atrium.6 Pathophysiology and Clinical Presentation: Small secundum ASDs may close spontaneously7; however, primum ASDs, sinus venosus septal defects, and coronary sinus defects generally do not decrease in size. Shunt volume is related to the size of the defect, right and left heart compliance, and pulmonary vascular resistance. With larger shunt volumes, right atrial, right ventricular, and pulmonary artery sizes increase. Over time, pulmonary hypertension may develop. Most infants and young children with ASDs are asymptomatic. ASDs usually are detected at about 6 months of age,8 often during evaluation for a murmur or as an incidental finding on a chest radiograph. Older children with moderate to large ASDs may have symptoms of fatigue and dyspnea. In addition to pulmonary hypertension, older children with ASDs may have atrial tachyarrhythmias or paradoxical strokes, a risk that increases with age.9–12 Imaging: Chest radiography in the neonate usually shows that the heart is normal in size and pulmonary flow is normal. Findings in infancy and childhood include mild cardiomegaly related to right atrial and right ventricular enlargement (Fig. 73-3). The left atrium is not enlarged, which distinguishes an ASD from other left-to-right shunt lesions. Usually main pulmonary artery enlargement and increased pulmonary vascularity is found if the pulmonary to systemic flow ratio is greater than two to one. If the patient has significant pulmonary hypertension, enlarged central pulmonary arteries and peripheral pulmonary arterial vessel tapering may be seen (e-Fig. 73-4). Figure 73-3 Atrial septal defect, secundum type, in a 7-year-old. Echocardiography is the imaging modality of choice to determine the location and direction of flow across the defect, to evaluate atrial and ventricular chamber size and ventricular function, and to assess for associated cardiovascular abnormalities. Magnetic resonance imaging (MRI) or computed tomography (CT) also may be used for evaluation of the atrial septum in cases of poor acoustic windows13 or to evaluate the pulmonary veins in patients with suspected sinus venosus defects (Videos 73-1 to 73-3). Left and right ventricular size and quantitative systolic function can be assessed, and right ventricular volume overload is detected as diastolic septal flattening or diastolic bowing of the septum from right to left with severe volume overload. Comparison of right and left ventricular stroke volumes can be used to calculate the ratio of pulmonary to systemic arterial flow (Qp : Qs). Right ventricular pressure is assessed by evaluating the degree of tricuspid regurgitation and septal systolic position. Systolic septal flattening is indicative of elevated right ventricular pressure. Phase contrast MRI can be used to estimate the size of the ASD and to determine the direction and amount of shunting at the atrial level by calculation of Qp : Qs (e-Fig. 73-5).14 Treatment: Closure should be performed in childhood to avoid complications of arrhythmia, right ventricular dysfunction, pulmonary hypertension, and paradoxical embolus.15 ASD closure can be performed surgically or via transcatheter closure; the latter procedure largely has become primary therapy for anatomically favorable secundum defects. After closure, children with ASDs have an excellent prognosis. Overview: Atrioventricular septal defects (AVSDs) account for 4% of all cases of congenital heart disease.16 Most patients with complete AVSD have Down syndrome.17 AVSD also is associated with the visceral heterotaxy syndromes.18,19 Etiology: AVSD results from abnormal development of the embryologic endocardial cushions and produces a spectrum of disease (Fig. 73-6). In its mild form or in partial AVSD, a crescent-shaped defect is found in the inferior portion of the atrial septum immediately adjacent to the atrioventricular (AV) valve, along with an associated “cleft” mitral valve with separate mitral and tricuspid valve orifices. Persons with the complete form have an ostium primum, a large inlet ventricular septal defect (VSD) beneath the plane of the AV valves, and a single or common AV valve, with variable leaflet size, location, and morphology. Most importantly, the common valve has two components that “bridge” the ventricular septum and may form attachments to the septal surface and/or both the right and left sides of the heart.20 In most cases, the common AV valve is shared equally between the right and left ventricles, but the valve orifice may be unequally shared and may favor either the right or left ventricle.19 Figure 73-6 Diagrams illustrating the spectrum of atrioventricular septal defect (AVSD). Pathophysiology and Clinical Presentation: With complete AVSD, the amount of left-to-right shunting is generally large but is related to the size of the ASD and VSD, right and left heart compliance, and pulmonary vascular resistance. The shunting may be interatrial or interventricular. The cleft mitral valve can lead to significant mitral insufficiency and can exacerbate congestive heart failure. Infants with complete AVSD present with tachypnea, tachycardia, and signs of congestive heart failure as pulmonary resistance falls during the newborn period. Patients with partial AVSD may be asymptomatic as infants and young children, although they may have symptoms earlier in life if significant associated mitral valve regurgitation is present.21 Imaging: The amount of left-to-right shunting is reflected on the chest radiograph and may reflect the physiology of either the ASD or VSD or both. With complete AVSD, findings include moderate to marked cardiomegaly with right atrial and right ventricular enlargement and increased pulmonary vascularity (Fig. 73-7). Left atrial enlargement may be seen if associated mitral insufficiency is present. Children with large left-to-right shunts commonly have lung hyperinflation, which may be related to an increase in airway resistance from enlarged pulmonary arteries and veins or to an increase in lung volume related to the increase in blood volume. Figure 73-7 Atrioventricular septal defect in a 6-week-old. Echocardiography is the primary modality for evaluation of AVSD, but MRI can be used as an adjunct in complex cases, especially when an accurate assessment of right and left ventricular size is needed. Components to evaluate include the ostium primum portion of the atrial septum and the inlet portion of the ventricular septum, the AV valve leaflet morphology and attachments, the papillary muscle architecture, the level and direction of shunting, ventricle size and systolic function, and the presence of outflow tract obstruction or other cardiovascular anomalies (Fig. 73-8).
Septal Defects
Atrial Septal Defect
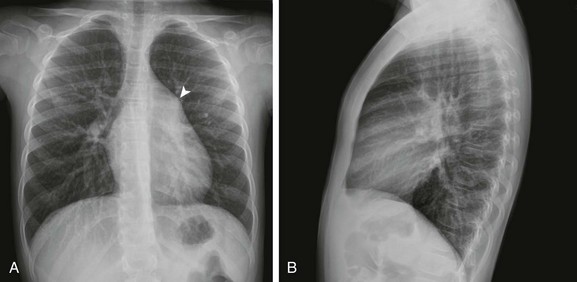
Frontal (A) and lateral (B) views of the chest show mild cardiomegaly, a prominent pulmonary artery (arrowhead), and increased pulmonary vascularity without left atrial dilation.
Atrioventricular Septal Defect
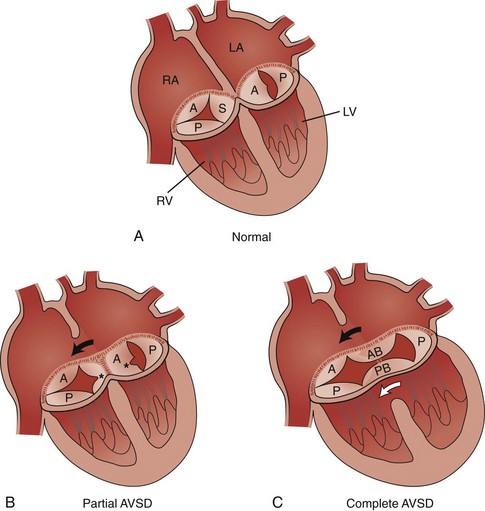
A, The normal tricuspid valve has three leaflets, anterior (A), posterior (P), and septal (S), and the normal mitral valve has two leaflets, anterior (A) and posterior (P). B, Partial AVSD showing the clefts (asterisks) in the septal leaflet of the tricuspid valve, the anterior leaflet of the mitral valve, and the ostium primum atrial septal defect (ASD) (arrow). C, Complete AVSD showing the common atrioventricular valve with the anterior bridging (AB) and posterior bridging (PB) leaflets, the ostium primum ASD (black arrow), and the inlet ventricular septal defect (white arrow). LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (Modified from Park MK. Left-to-right shunt lesions. In Park MK, editor: Pediatric cardiology for practitioners, St. Louis: Mosby Elsevier; 2002.)
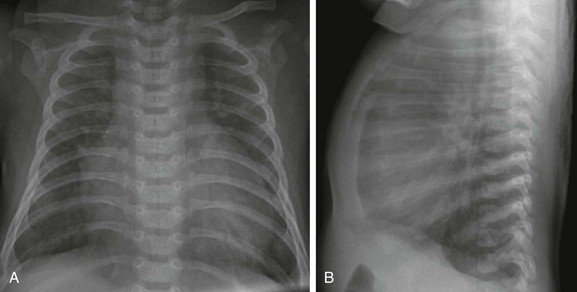
Frontal (A) and lateral (B) views of the chest show moderate cardiomegaly with enlargement of the right atrium and ventricle, increased pulmonary vascularity, and lung hyperinflation.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree