Chapter 45 Overview: Spinal tumors occur predominantly in young or middle-aged adults and tend to be less common in children. The most common presentation is 3 to 6 months of pain, gait disturbance, change in spinal curvature, motor weakness, and bowel and bladder dysfunction. In the setting of acute trauma, peritumoral edema may cause paresis or paralysis. In this chapter, general concepts regarding spinal neoplasms will be discussed, followed by specific anatomic categories of lesions. Categories will include neoplasms located in the intramedullary, intradural extramedullary, or extradural compartments.1 Imaging: The imaging features of spinal neoplasms are often nonspecific, and findings overlap. Familiarity with age at diagnosis, key imaging findings, and associations can narrow the differential diagnosis, as summarized in Table 45-1. Intraoperative ultrasound may be used to determine tumor location and borders during exposure and resection. Baseline postoperative MRI is deferred for at least 12 weeks because surgical changes make early postoperative scans difficult to interpret.2 Treatment: The goal with benign, noninfiltrative spinal neoplasms is complete excision of both the solid tumor and associated syrinx cavities. If any portion of the neoplasm infiltrates the cord, surgical success decreases. Adjuvant radiation therapy and chemotherapy may be used, but the prognosis with an infiltrative, malignant lesion is dismal.3 Typically, a laminectomy is performed to gain access to the spinal cord lesion. Myelotomies are performed in the midline between the posterior columns or along the dorsal root entry zone. The tumor must be centrally debulked. Electrophysiologic monitoring techniques can be helpful as the periphery of the tumor is approached to prevent damage to the lateral columns of the spinal cord. The wound is closed when the tumor has been removed to its interface with normal-appearing spinal cord and the associated syrinx cavity has been carefully inspected for additional tumor deposits.3 Prognosis: Preoperative neurologic deficits may persist postoperatively, but most tumors can be removed without new long-term disabilities. If complete tumor excision is not possible with histologically benign pediatric intramedullary gliomas, adjuvant radiation therapy is preferentially avoided because of adverse effects on the immature spinal cord and spinal column. Residual and recurrent tumor deposits often remain static for years or grow very slowly. Symptomatic recurrences can be managed by operating again. Surgical management is considered with extradural tumors to establish a pathologic diagnosis with biopsy, to decompress the spinal cord in the setting of progressive myelopathy, for reconstruction in cases of spinal instability, and with an attempted radical resection for cure. Most childhood spinal epidural lesions are responsive to radiation or chemotherapy.4 Overview: Pediatric intramedullary tumors occur most commonly in the cervicothoracic cord.5 Nonspecific symptoms often lead to a delay in diagnosis and vary with the age of the child. Younger children may present with spinal pain (dull and aching) or root pain, rigidity, persistent unexplained torticollis, and muscle spasm. Older children may present with gait disturbance and/or progressive scoliosis. Extremity weakness and paresthesias are common as well.6 A subset of patients may present with symptoms of increased intracranial pressure (ICP) and hydrocephalus. Overview and Origin: Up to 60% of intramedullary tumors in children are astrocytomas, and the cervical cord is most commonly involved.3 Spinal astrocytomas usually occur in children around 10 years of age, with an equal sex predilection. Spinal astrocytomas are rarely seen in neonates and infants and may present with irritability, torticollis, and loss or absence of developmental milestones. Symptoms often are protracted.6 Astrocytomas arise from astrocytes and range from benign (grade I) to malignant (grade IV).7 Spongioblastomas and pilocytic astrocytomas are at the benign end of the spectrum, and high-grade astrocytomas and glioblastoma multiforme tumors are at the malignant end of the spectrum. Spinal astrocytomas may be cystic, mixed cystic and solid, solid, or contain necrotic components. Malignant astrocytomas can mimic spinal vascular malformations as a result of hypervascularity with possible intratumoral hemorrhage. Lesion size varies from focal to involvement of the entire spinal cord (a holocord tumor), which usually is seen during the first year of life.8 Imaging: Contrast-enhanced spinal MRI is critical to the identification of small tumors with associated syringohydromyelia and to detect subarachnoid seeding. Key features of astrocytomas include cord expansion, eccentric location, hypointense to isointense appearance on T1-weighted imaging, heterogeneous hyperintensity on T2-weighted imaging, and variable enhancement, sometimes of a mural nodule, on postcontrast images (Figs. 45-1 and 45-2). T2 heterogeneity depends on the presence of solid, cystic, and necrotic components.5 Figure 45-1 An astrocytoma in an 8-year-old boy with back pain. Figure 45-2 An astrocytoma in a 6-year-old boy with pain and decreased use of his right arm. Overview and Origin: Up to 30% of intramedullary tumors in children are ependymomas. Ependymomas present in an older age group than do astrocytomas (at age 13 or 14 years), with a slight female predilection.3 Ependymomas originate from ependymal cells within the central spinal cord or filum terminale, and they frequently span multiple segments. Holocord involvement, as with astrocytomas, is possible.9 A variety of histologic subtypes of ependymomas exist, with cellular ependymoma the most common. Myxopapillary ependymoma occurs exclusively in the lower cord and filum terminale. Surgical management of the myxopapillary subtype is difficult because of varied physical consistency of the lesion and relationship to surrounding structures. When occurring in the cauda equina, this subtype may be associated with subarachnoid hemorrhage, back pain, lower extremity weakness, numbness, pain, and even bowel and bladder incontinence.10 Imaging: Ependymomas are distinguished by the findings of central cord location, cord expansion, heterogeneous signal intensity on all sequences, and a T2 hypointense hemosiderin cap along the cranial or caudal aspects of the tumor (Figs. 45-3 and 45-4). Contrast-enhanced spinal MRI is useful in the evaluation of small drop metastasis, a common feature of ependymomas.11,12 The presence of metastatic spread to the brain with resultant hydrocephalus can be evaluated with cranial MRI. Multiple ependymomas should prompt consideration of neurofibromatosis type 2 (NF2).13 Figure 45-3 An ependymoma in an 11-year-old boy with unexplained altered mental status. Figure 45-4 A myxopapillary ependymoma in an 11-year-old girl with back pain. Overview and Origin: Gangliogliomas contain both astrocytic and neuronal components. They present in the first three decades with an average presentation age of 12 years.5,14 Imaging: Gangliogliomas may be solid, cystic, calcified, or hemorrhagic. They demonstrate heterogeneous signal intensity on all sequences.15 Notable imaging features include a lack of peritumoral edema, even when large in size, and associated adjacent osseous erosion (Fig. 45-5). Given this lesion’s similar appearance to an ependymoma on MRI and its association with patients who have NF2, it should be included in the differential diagnosis when ependymoma is a diagnostic consideration.14 Figure 45-5 Ganglioglioma.
Spinal Tumor and Tumorlike Conditions
Spinal Neoplasms
Intramedullary Neoplasms
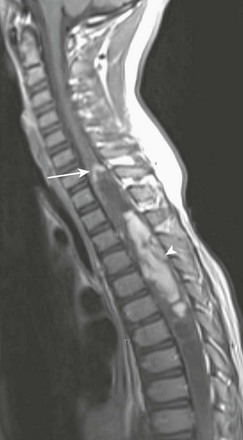
A sagittal T1-weighted contrast-enhanced image demonstrates an intramedullary cystic mass with dorsal nodular (arrowhead) and peripheral cranial (arrow) enhancement.
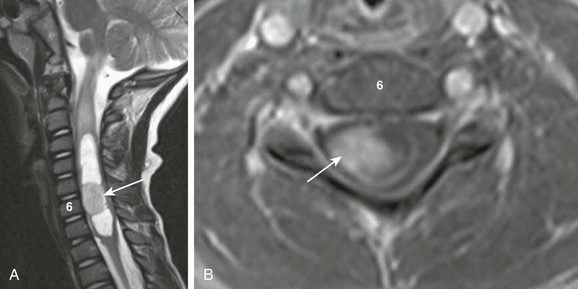
A, A sagittal T2-weighted image shows an intramedullary cystic cervical cord mass with an enhancing mural nodule (arrow). Note T2 bright cord edema above the mass. B, An axial T1-weighted image with contrast through the enhancing nodule shows that the lesion is eccentric.
Ependymoma
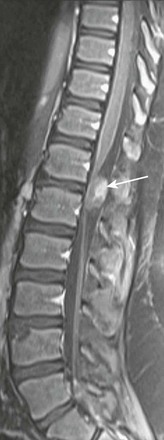
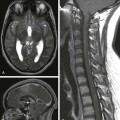
A sagittal T1-weighted image with fat suppression demonstrates an extramedullary heterogeneously enhancing mass (arrow) at T12-L1 displacing the nerve roots ventrally.
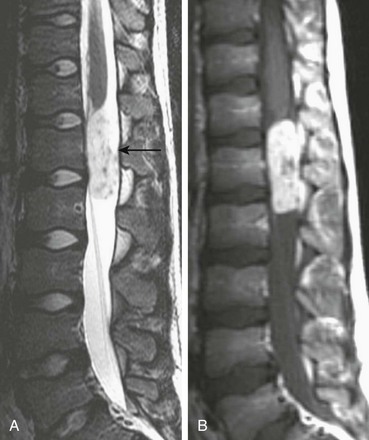
A, Sagittal T2-weighted imaging demonstrates a hyperintense, well-defined mass with internal flow voids arising just below the conus medullaris (arrow). B, A sagittal contrast-enhanced T1-weighted image confirms a mass and shows avid enhancement.
Ganglioglioma
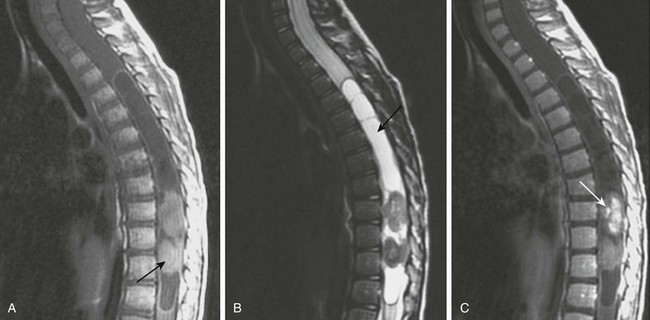
A, A sagittal T1-weighted image shows a holocord tumor. Rounded hyperintense elements seen at the thoracolumbar junction were found at the time of surgery to represent hemorrhagic components of the tumor (arrow). B, A sagittal fat-suppressed T2-weighted magnetic resonance (MR) image shows the hemorrhagic components of the lesion as hypointense masses. Note the tumor cyst in the midthoracic cord (arrow). C, A sagittal postenhanced T1-weighted MR image shows enhancement of the caudal tumor (arrow).