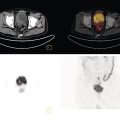
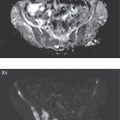
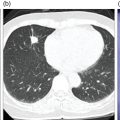
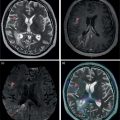
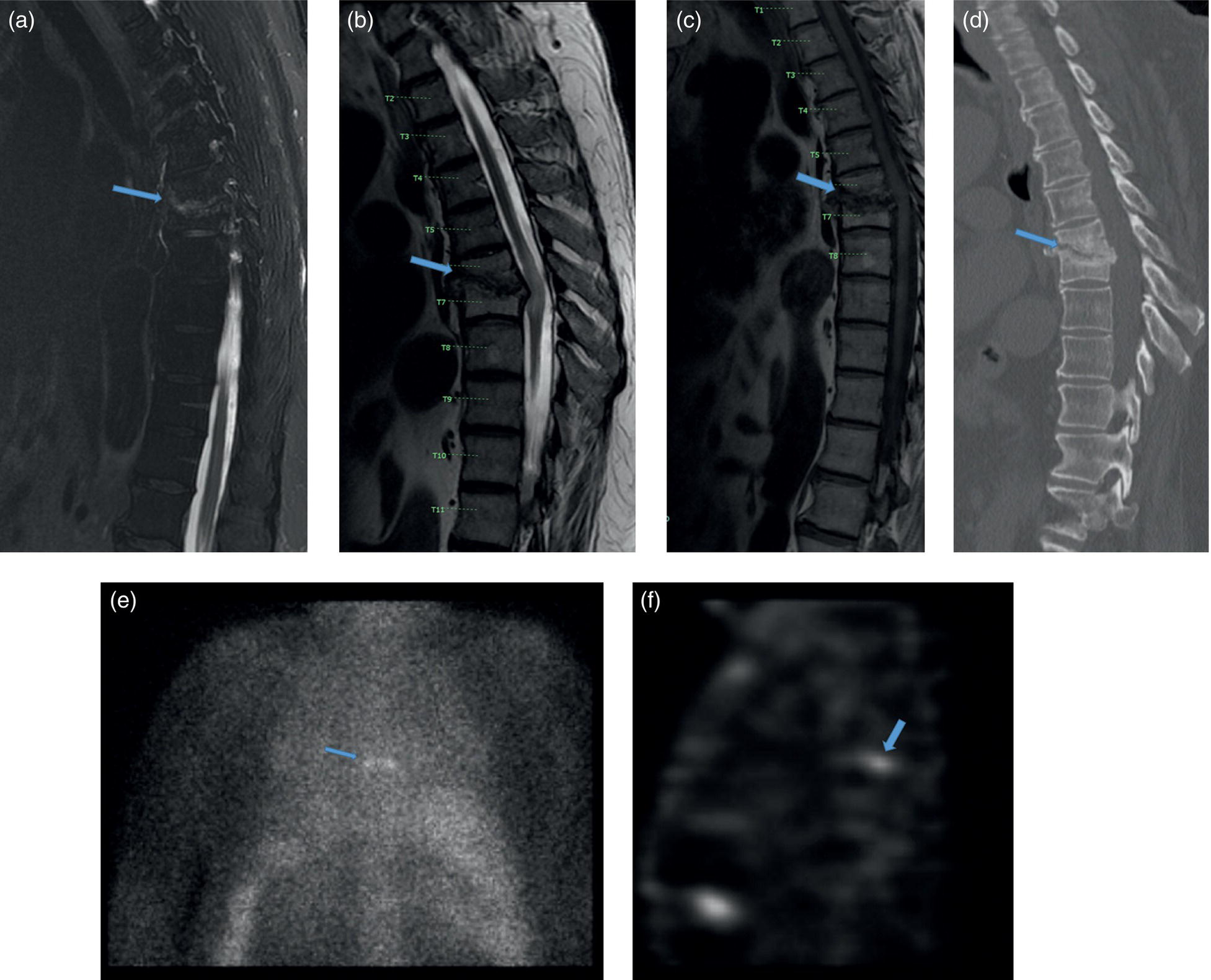
Azadeh Eslambolchi1, Amit Gupta2,3,4, Jay Acharya5, Christopher Lee6, and Kaustav Bera7 1 Pediatric Radiology Section, Mallinckrodt Institute of Radiology, Washington University in St Louis, School of Medicine, St. Louis, MO, USA 2 Radiology, Medicine and Biomedical Engineering, Case Western Reserve University School of Medicine, Cleveland, OH, USA 3 Cancer Imaging Program, Case Comprehensive Cancer Center, Cleveland, OH, USA 4 Diagnostic Radiography, University Hospital Cleveland Medical Center, Cleveland, OH, USA 5 Radiology, Keck School of Medicine of USC, HCCII Lower Level Radiology, Los Angeles, CA, USA 6 Keck School of Medicine of USC, HCCII Lower Level Radiology, Los Angeles, CA, USA 7 Case Western Reserve University School of Medicine, University Hospital Cleveland Medical Center, Cleveland, OH, USA During the past few decades, spinal disorders, such as degenerative disease, infections, and tumors, have been globally increasing in all age groups [1]. To address these health concerns different imaging modalities have stepped into the picture. The recent technological developments have improved our diagnostic capabilities in varied spinal pathologies. The spinal column has a complex anatomy with various soft tissue and osseous components, demanding more precise and accurate imaging assessment [2]. Plain radiography was used as the first diagnostic modality to assess bony elements from the early days of X‐rays. In the mid‐1970s, computed tomography (CT) broke into the arena by producing two‐ and then three‐dimensional images of the spine. Not long after, the introduction of magnetic resonance imaging (MRI) was another great leap forward, boasting exquisite contrast resolution and radiation‐free visualization of the spine anatomy [3]. Around the same time, radionuclide bone imaging came into use and became a common investigatory modality in most nuclear medicine departments. The range of available radionuclide tracers gradually expanded, especially with the increased availability of positron emission tomography (PET) in later years. All imaging modalities have their own advantages and drawbacks based on their mechanisms and principles. In recent years, to address the limitations of each modality and to better characterize lesions, CT and MRI have sometimes been used in conjunction with nuclear medicine techniques, including single‐photon emission computed tomography (SPECT) or PET scanning. Hybrid systems of both modalities have also been designed to leverage the benefits of each modality while overcoming their shortcomings to increase the overall diagnostic sensitivity and specificity. The combination of these two modalities may be of great help in diagnosing cases with ambiguous presentations on a single modality. Traces of infection ruining the human spine have been detected in excavations dating back to 7000 BC [4]. Indications of spine infection has been demonstrated in Egyptian mummies [5] and skeletal remains excavated in South‐East Asia [6]. Hippocrates and Galen both explained spinal infection and its relation to spinal deformity. Pott recounted tubercular infection of the vertebral column in 1779 and Lannelonge discussed bacterial spine infection in France in 1879 for the first time [7]. Spinal infection can be classified according to the location of the involvement in intraspinal (intramedullary, subdural, or epidural), bone, disc, and paraspinal (e.g. psoas abscess) infections [8]. If infection involves the intervertebral fibrocartilage, it is referred to as discitis [9], and if the vertebral body or the end plate is infected, it is labeled as spondylitis or vertebral osteomyelitis [10]. Nevertheless, the diagnosis and treatment of both these entities are similar, and usually both the structures are involved at the time of diagnosis, therefore are discussed together as spondylodiscitis. Three main modes of pathogen transmission to the spine have been described. It can be disseminated hematogenously from somewhere far in the body, by contiguous spread from adjacent tissues or exogenously through direct external inoculation, mostly by spinal surgeries, and rarely by trauma or intraspinal injections. Infection can less commonly derive from lymphatic vessels [11]. Hematogenous spinal infection mostly affects lumbar spine, followed by thoracic and cervical spine [12]. By contrast, tuberculosis mainly involves thoracic vertebrae, often involving two levels or more, which can be considered as a diagnostic clue, compared to pyogenic infection [13]. Intervertebral discs are affected secondary to neighboring endplates, necrotized by pyogenic infection [14]. Infection in the vertebral column can extend far beyond the bony structure and appear as a psoas or other soft tissues abscesses. The opposite spread of infection from adjacent tissues to the spine can rarely happen. This can be from neighboring infections, such as infected implants, retropharyngeal abscess or esophageal rupture [15]. The frequency of spinal infections has increased during the past few decades. The advent of more efficient and sensitive imaging modalities has led to more accurate and early diagnosis of the disease. An increasing section of the population going through senectitude can be another considerable cause for this increase [16], not to mention the ever‐increasing number of spinal procedures and instrumentations [17]. Furthermore, comorbidities, such as diabetes, obesity, intravenous drug abuse, prolonged steroid consumption, chronic infection (particularly HIV), immune deficiency, and poor nutrition, can all serve as risk factors for spinal infection [18]. A variety of microbes, including bacteria, fungi, and parasites, have been implicated in infections of the spinal column. Pyogenic spine infection is caused by bacteria and the majority of granulomatous infections by fungi, mycobacterial tuberculosis, and Brucella species. Tuberculosis was once the most common cause of spinal infection. Due to considerable decline in the incidence of pulmonary tuberculosis, spinal tuberculosis has also decreased noticeably in the past 50 years, although it is still fairly common worldwide, particularly in HIV‐positive patients. Currently, the most common organism responsible for spinal infection is Staphylococcus aureus and the infection is often mono‐microbial [19]. Other microorganisms also can be responsible, including but not limited to coagulase‐negative Staphylococci, Gram‐negative bacteria, such as Escherichia coli, and anaerobic agents, especially in the setting of penetrating trauma [20]. In endemic areas of tuberculosis and brucellosis, like the Mediterranean region, Latin America, the Middle East, parts of Africa, and Eastern Europe, spinal infection due to these organisms is still quite prevalent [21]. Fungal infection of the spine is rare and mainly results from Aspergillus species, Candida species, and Cryptococcus neoformans. In spite of great progress in the identification of responsible pathogens, the infectious agent still cannot be found in almost one‐third of patients [8, 22]. Infections of the spinal column commonly yield a nonspecific and ambiguous clinical picture, especially in the early phase of the disease, making early detection of the infection challenging and onerous [23]. Back pain is the most common complaint, followed by fever and neurologic impairment. Spinal canal (intramedullary, subdural, and epidural) and paravertebral (psoas) abscesses are among common complications of spinal infection and may lead to neurological deficits, even paraplegia in extreme cases [24]. A considerable number of patients complain of continuing pain and spinal dysfunction even after recuperation [25]. A plain X‐ray is generally obtained as an initial step for evaluating any possible pathology in the spine. Radiographs are of low sensitivity and specificity in spinal infection, particularly in the early stages, but may help exclude other possible diagnoses. Bone destruction needs to exceed 30% to be detectable in X‐rays and this may take several weeks to happen; however, early radiographic changes may appear as cortical bone haziness and endplate erosion in the anterior corner, and can spread to the entire endplate. Due to the fragmentation of the intervertebral disc in later stages, the disc space loses its height, leading to intervertebral disc narrowing. Osteolysis, subchondral bone destruction, and paravertebral swelling may also be seen [26]. As the infection progresses, signs of bone reformation may appear as osteophytes and peripheral sclerosis. All these may lead to vertebral collapse and spinal deformity. Malalignment in sagittal and coronal planes secondary to these damages can be detected in anteroposterior and lateral views. Potential instabilities can be evaluated by utilizing flexion and extension views. CT is of limited use in the diagnosis of spinal infection and may be normal in the early stages. The delay of 3–6 weeks for bone destruction to appear on CT has resulted in lower diagnostic yield in early stages of infection [27]. Narrowing of the disc space in CT is also nonspecific and more commonly seen with osteoarthritis. However, CT is still the best imaging modality for detection of endplates changes (much before becoming visible on X‐ray), bone necrosis, and revealing calcifications suggestive of tuberculosis. While MRI is more useful for evaluation of the central canal and the spinal cord, CT visualizes bony changes better and can be considered as a supportive modality to MRI. CT can be particularly useful for evaluating bone necrosis, which may require surgical debridement. When MRI is contraindicated for any reason, contrast‐enhanced CT (CECT) can be obtained instead. By the use of contrast media, enhancement in soft‐tissue inflammation and abscess can be delineated [28]. CECT can detect paraspinal abscess formation, with obliteration of fat planes and soft tissue swelling. CT is also generally used in the context of percutaneous CT‐guided biopsy and percutaneous abscess drainage [29]. MRI is currently the modality of choice for the diagnosis of spinal infection [30]. It has a high sensitivity (82–96%), specificity (85–92%), and accuracy (81–94%) [31], and yields comprehensive information of epidural space and soft tissues around the spine [31]. This is related to direct multiplanar imaging capability, high tissue contrast resolution, and better sensitivity for soft tissue and bone marrow abnormalities. As disc infections spread quickly to neighboring endplates, both disc and vertebral body characteristically elicit a hypointense signal on T1‐weighted and hyperintense signal on T2‐weighted images due to edema. Post‐contrast MRI images after gadolinium injection show enhancement in vertebral body, intervertebral discs (diffuse, patchy or linear), and surrounding soft tissue [32]. This increases the accuracy of MRI and helps in differentiating infective spondylodiscitis from degenerative [30] and tumoral changes (in tumors, hypointensity and enhancement are limited to the vertebral bone marrow and are not seen in the discal space) [33]. However, spinal infection has no pathognomonic findings on MRI to differentiate it from other pathologies. MRI is also of great help in distinguishing spinal tuberculosis infection from pyogenic spondylitis. Bone destruction in tuberculosis (TB) spondylitis is usually extensive, while the intervertebral disc is usually spared. The vertebral body shows heterogeneous enhancement, and large paravertebral abscesses (with smooth and thin rim enhancement) are more common with TB [34]. Tuberculosis infection can extend beneath anterior longitudinal ligament, caudally or cranially. This can be more extensive than vertebral involvement, manifest as anterior scalloping of the vertebral body, and result in a large subligamentous abscess. Posterior elements can be involved in TB spondylitis and skip lesions are also more common [35]. MRI is contraindicated in the presence of cardiac pacemakers and certain metallic implants in the spine. Although spinal implants are mostly MRI‐compatible nowadays, implants may still cause artifacts and degrade image quality [36]. Another disadvantage of MRI is related to the overestimation of the amount of infected tissue, as the surrounding reactive tissue also shows the same signal changes. MRI is less sensitive in diagnosing spondylodiscitis, if imaging is performed in the early stages of the disease (within 2 or 3 weeks of symptom onset) [37]. In spite of increasing use of MRI to oversee treatment responses in spondylodiscitis, MRI changes may persist and even worsen during antibiotic therapy. This may happen despite clinical improvement and may result in unnecessary invasive treatments [38]. All these call for complementary diagnostic protocols. Bone scintigraphy is frequently used as a screening test for infection. However, coexisting conditions, such as fracture and orthopedic hardware, reduce its specificity. Furthermore, 99mtechnetium (99mTc) and 111indium (111In) white blood cell (WBC) scans have still lower sensitivity in spinal infection. In practice, 111indium WBC scan is not recommended for assessing suspected spinal involvement as it can show infection as nonspecific areas of decreased activity in vertebrae and cannot differentiate infection from other causes of marrow replacement. On the contrary, 67gallium (67Ga) SPECT can be a reliable alternative for MRI and is currently the radionuclide procedure of choice for spinal infection [39]. It can be performed when MRI is contraindicated or when the diagnosis is inconclusive. However, 67Ga can accumulate in traumatic tissues, tumors, and noninfectious inflammations other than infectious areas, resulting in false‐positive results. Additionally, the required 1–3 days of interval between injection of 67Ga and image acquisition delays the diagnosis. More recently, 111In‐biotin SPECT/CT has shown similar sensitivity compared to SPECT, and accurately differentiates between bone and soft‐tissue infection [40]. The positron‐emitting gallium isotope 68Ga offers certain advantages over 67Ga for diagnosing infections. However, there are still limited data available on the role of 68Ga imaging in spinal infection [40]. Relative low specificity of SPECT has resulted in an increasing interest in 18F‐labeled fluorodeoxyglucose (18F‐FDG) PET and combined 18F‐FDG positron emission tomography and computed tomography (PET/CT) as a promising technique in the diagnostic workup [41], which is gradually replacing SPECT as the radionuclide modality of choice for spinal infection. Hybrid imaging has brought about an appreciable benefit to the field of nuclear medicine. In PET/CT, functional and anatomical information are acquired in a single gantry at the same time without moving the patient. The co‐registered images have improved diagnostic capability and accuracy significantly, especially in patients with musculoskeletal and spinal infection. This technique facilitates differentiating bone and soft tissue infections. The role of 18F‐FDG for diagnosing spinal infection has been extensively investigated [42]. A large‐scale study to compare the diagnostic accuracy of 18F‐FDG PET/CT and MRI is not available yet. However, several small studies have compared the accuracy of these two modalities during past few years. Some of these studies suggest superiority for 18F‐FDG PET/CT while others report equivalent performance of 18F‐FDG PET/CT and MRI in diagnosing spondylodiscitis. Note that the low sensitivity of MRI in some studies can be due to employing this modality in early stages of the infection [43]. It has been suggested that in the first 2 weeks of infection due to low sensitivity of MRI, 18F‐FDG PET/CT has superior diagnostic utility in spondylodiscitis and might have a higher sensitivity (up to 100%) than MRI. After 2 weeks, similar results can be seen with both techniques [44]. This can be explained by inherent differences between the two modalities: while MRI explores anatomic changes, 18F‐FDG PET captures changes in glucose metabolism, which begins to increase in the very early stages of the infection [45]. In patients with Gram‐positive bacteremia, 18F‐FDG PET/CT has been shown to be cost‐effective [38] and nowadays many patients presumed to have spinal infection undergo this procedure. MRI is accepted as the imaging modality of choice in diagnosing epidural and spinal abscess [30], while 18F‐FDG PET/CT has been negative in a considerable number of these cases. On the contrary, the sensitivity of 18F‐FDG PET/CT has been shown to be higher in diagnosing paravertebral and psoas abscesses [46]. In a study by Smids et al., MRI could depict all the cases of epidural and spinal abscess, while 18F‐FDG PET/CT was negative in almost half of these patients; however, in diagnosing paravertebral abscess, 18F‐FDG PET/CT showed higher sensitivity than MRI [44]. Type I vertebral degeneration and spondylodiscitis exhibit similar signal changes in MRI as low intensity in T1 and high in T2‐weighted images, and telling them apart is a challenge on conventional MR sequences. With the help of diffusion‐weighted MRI (DW‐MRI) and the presence of the claw sign (a well‐defined linear high signal at the interface of infective and normal bone marrow due to hyper‐cellularity and pus‐related diffusion restriction), the differentiation can become more feasible [47]. Still, 18F‐FDG PET/CT has been shown to outperform MRI in differentiating spondylodiscitis and degenerative disease [43]. In 18F‐FDG PET/CT, imaging of the whole body provides the possibility of identifying septic foci elsewhere in the body, even identifying endocarditis, which can happen concurrently with spondylodiscitis [48]. Preliminary results have shown that 18F‐FDG PET/CT may be a promising modality for evaluation of treatment results, while, as mentioned, MRI is not the best modality for this purpose [49]. In the evaluation of the infection in the postoperative situation and the presence of metallic devices, 18F‐FDG PET is more sensitive than MRI and CT, as 18F‐FDG uptake is not impeded by metallic implant‐associated artifacts [50] (Figure 22.1). Due to the complex structure of the vertebral column and overlapping imaging characteristics of many spinal lesions, the diagnosis of spinal tumors can be demanding and burdensome. Accurate early diagnosis of these tumors by choosing the best imaging modalities can preclude unnecessary and invasive diagnostic procedures, leading to best treatment choices and deferring undesired complications, such as mass effects on the thecal sac and irreversible neurological outcomes due to cord compression [51]. Age and tumor location (posterior elements vs. vertebral body) can help distinguish different types of tumors. Generally, with the exception of osteosarcoma and Ewing sarcoma, most spinal lesions that appears before 30 years of age are considered benign [52]. The most common lesions centered within the vertebral body are hemangiomas and enostosis (bone islands). Other lesions within the vertebral body can be multiple myeloma (MM), plasmacytoma, giant cell tumor (GCT), lymphoma, eosinophilic granuloma (EG), and chordoma [53]. Lesions with primary involvement of posterior elements are generally benign, including osteochondroma, osteoma, aneurysmal bone cyst (ABC), and osteoblastoma. Malignant lesions, including osteosarcoma, Ewing sarcoma, and chondrosarcoma, originate from the vertebral body, but may involve posterior elements in advanced cases. Both malignant and benign lesions, such as osteoblastomas or even vertebral hemangiomas, can expand and compress the spinal cord and nerve roots, and result in neurological symptoms [54]. Figure 22.1 A 71‐year‐old male presented with subacute back pain. Sagittal short tau inversion recovery (STIR) (a), T2 weighted (b), and precontrast T1‐weighted (c) images of the thoracic spine demonstrate compression deformity of T6 and T7 vertebral bodies with obliteration of the intervertebral disc, as well as Modic type 1 endplate changes (marrow edema) at the T6–T7 level (blue arrows), concerning for spondylodiscitis, however, degenerative and traumatic etiologies are also differential considerations. The sagittal CT image of thoracic spine (d) better shows the extent of osseous destruction of the inferior endplate of the T6 vertebral body and superior endplate destruction (d, arrow) along with focal kyphosis at this level, again suggestive of osteomyelitis discitis. Gallium scintigraphy, posterior planar (e) and sagittal SPECT (f) images demonstrate focal radiotracer within the thoracic spine (blue arrows), which correlates with CT and MRI findings at T6/T7 level, confirming osteomyelitis/discitis. Some distinguishing imaging characteristics also help differentiate malignant from benign lesions. Benign tumors generally show well‐defined borders while aggressive lesions demonstrate a wider zone of transition. Malignant tumors also exhibit a more aggressive periosteal reaction, such as lamellated or sunburst features. On MRI, aggressive lesions commonly show adjacent marrow edema, a soft tissue component, and edema. Moreover, some lesions demonstrate specific radiologic characteristics. A sclerotic lesion with a central nidus is specific for osteoid osteoma. Lesions plainly containing fat are generally benign, the majority being hemangiomas [53]. Fluid–fluid level on MRI or CT is most commonly associated with ABC; however, it can also be seen with GCT, telangiectatic osteosarcoma, and chondroblastoma (although rarely seen in the spine). In hemangiomas the primary vertical weight‐bearing trabeculae show thickening on CT, causing the corduroy sign on sagittal views and the polka dot sign on the transverse plane. CT and MRI can be used in conjunction with PET to better characterize spinal lesions. Generally, spinal malignancies are more avid on 18F‐FDG PET/CT than benign tumors [55]. 18F‐FDG PET/CT is a worthy tool to distinguish metastatic lesions from benign spinal tumors, and is more specific and sensitive compared to CT or MR imaging alone [56]. Cartilage lesions may present with characteristic features on CT and plain X‐ray, and are high T2 signal on MRI; however, these characteristics do not necessarily differentiate benign and malignant lesions. On the other hand, 18F‐FDG PET has 100% specificity and 91% sensitivity in distinguishing chondral lesions with maximum standardized uptake value (SUVmax) greater than 2 for malignant lesions and SUVmax of lower than 2 for benign lesions [57, 58]. Enchondroma, although benign, shows a rather high 18F‐labeled sodium fluoride (18F‐NaF) PET uptake due to its osteoblastic activity, causing confusion in distinguishing it with chondrosarcoma [59]. Benign tumors can exhibit widely varying degrees of 18F‐FDG avidity. Several vertebral tumors, such as ABC, fibrous dysplasia, EG, osteoblastoma, and osteoid ostoma, can show considerable 18F‐FDG uptake [60]. Some of these lesions may exhibit the same 18F‐FDG avidity as malignant tumors, such as Ewing sarcoma. Intraosseous lipomas, benign cartilaginous lesions, and hemangiomas are the only benign spinal lesions with reliably low 18F‐FDG uptake. Therefore, 18F‐FDG and 18F‐NaF PET when combined with MRI and CT can be diagnostically helpful in certain instances. In a study of 150 lesions in 20 patients by Lansteger et al., 18F‐FDG PET/CT proved to be better at detecting osteolytic lesions while 18F‐NaF PET/CT was better at detecting non‐18F‐FDG‐avid lesions, such as renal cell carcinoma and thyroid cancer [61]. Other studies have shown 18F‐NaF to be superior in evaluating sclerotic lesions [62]. Osteoid osteoma is a primary and benign bone‐producing lesion. Henry L. Jaffe, the American pioneer in the history of bone pathology, first described this osteoblastic tumor as a unique entity in 1935 [63]. Osteoid osteoma accounts for approximately 10% of all benign skeletal tumors [64]. It can appear in any bone in the body, but is most commonly seen in long bones. Spinal involvement happens in 10–20% of cases [65]. In some studies, up to 40% spinal involvement has been described [66]. Typically, patients are under 25 years of age and men are more likely to be affected [67]. In the vertebral column, osteoid osteoma has a predilection to the lumbar region [68]. It is found more in the vertebral arch and is rarely seen in the vertebra body [69]. Pars interarticularis is the most common (73%) site of involvement on the vertebral arch [70]. Osteoid osteoma can also involve the lamina and pedicles on the vertebral arch. Spinous and transverse process involvement have also been reported, although less commonly. Osteoid osteoma is not locally aggressive and there is no potential for malignant transformation. An osteoid osteoma presents with a nidus of vascular osteoid surrounded by sclerotic bone. Osteoid osteomas are approximately 10–15 mm in diameter and do not exceed 2 cm [67]. A lesion greater than 2 cm is classified as osteoblastoma. Nerve fibers have been discovered within the nidus by special immunohistochemical techniques and are concentrated within the area of sclerosis surrounding the nidus [71]. Osteoid osteoma’s etiology is still unresolved. Some believe that it could be due to an inflammatory process or trauma, while others consider it a benign neoplasm. A history of trauma has been reported in one‐third of patients; nevertheless, further research is warranted to elucidate the correlation [72]. Microscopically, the nidus consists of interconnected and well‐organized trabecular bone in a background of vascularized fibrous connective tissue [73]. Stiff and painful scoliosis, nocturnal pain, and nerve root irritation are classical clinical findings of osteoid osteomas in the vertebral column [69]. Back pain is an uncommon complaint in young adults and adolescents, therefore when it appears in this population and accompanied by paravertebral scoliosis and muscle spasm, osteoid osteoma should be considered as an important differential consideration [74]. In fact, osteoid osteoma is the most common cause of painful scoliosis in adolescents [75]. The pain is thought to be induced by prostaglandin secretion inside the nidus [75] and is typically subsided with acetylsalicylic acid [67]. Painful scoliosis is due to muscle and soft tissue irritation, and finally results in asymmetrical spasm [76]. Two‐thirds of spinal osteoid osteomas present as painful scoliosis [15] and diagnosis should be considered in any case of atypical scoliosis (right convex lumbar scoliosis or left convex thoracic scoliosis). Torticollis, hyper lordosis, and kyphoscoliosis may also accompany this lesion [77]. As in any other bone tumor, plain radiograph is the primary imaging modality of choice in osteoid osteoma. On X‐ray, it is classically a small, round, radiolucent nidus, surrounded by a dense fusiform reactive sclerosis. The nidus itself may contain areas of calcification due to varying amounts of mineralized tissue [67], and it may not be detected in plain X‐ray due to the superimposition of surrounding sclerosis. Spinal radiographs particularly can miss the tumor because of their small size and complex anatomy of the vertebral column [78], particularly in the thoracic region. Nevertheless, every patient with scoliosis should receive a thorough radiographic evaluation at presentation, especially when back pain is also present. In most cases of osteoid osteomas in the spine, plain X‐ray appears as an area of neural arch sclerosis at the apex of the concavity of a scoliosis [69]. Other signs in plain radiograph include regional osteoporosis, and solid or laminated periosteal reaction. In cases where plain radiographs are not conclusive but clinical suspicion is high, further imaging workup will be requested [79]. Thin‐slice CT is an imaging modality which is highly sensitive for detecting the lesion and specifying the location of osteoid osteoma. The location can be cortical, medullary or subperiosteal. Osteoid osteoma on CT typically presents as a small, rounded target‐shaped nidus, with low attenuation [80]. The nidus can contain mineralization which can be ring‐like, amorphous, or punctate. Surrounding reactive sclerosis can vary from mild sclerosis to extensive periosteal reaction and new bone formation that may conceal the nidus [81]. Spinal osteoid osteoma is also better seen in CT. The nidus is visible as a low‐density area in posterior elements. Surrounding sclerosis of the ipsilateral pedicle, lamina, or transverse process may be present. According to Touraine et al., the nidus mineralization ratio in osteoid osteomas increases significantly with pain duration and can be regarded as a marker of the age of the tumor. In this study, no association between nidus size and pain duration was found, however [82]. Dynamic CECT may help distinguish an osteoid osteoma, which has a hypervascular nidus, from a Brodie’s abscess or a fluid‐containing simple bone cyst, which are nonvascular [83]. CT scanning before surgery has been shown to be helpful for determining the location of the tumor and the precise extent of osseous involvement [84]. Osteoid osteoma typically has an avid and early uptake on triple‐phase scintigraphy [85]. Scintigraphy used to be the preferred modality for the diagnosis of osteoid osteoma before the widespread use of CT imaging. It is the most sensitive tool for tumor localization, with 100% sensitivity [86]. In three‐phase skeletal scintigraphy, osteoid osteoma is characterized with an intense, round activity at the nidus, surrounded by less intensity reactive bone, known as a double density sign [87]. The increased intensity of the nidus is the result of increased bone turn over. The less intense peripheral radiotracer uptake represents the host bone tumor response. However, because of less peripheral sclerosis in vertebral body, this sign is rarely seen with spinal osteoid osteoma [88]. MRI appearance in osteoid osteoma varies according to the size of the fibrovascular zone, the amount of calcification within the nidus, and the amount of reactive sclerosis and bone marrow edema [89]. The nidus in osteoid osteoma has a variable and highly heterogeneous appearance on MRI, and its characterization is generally difficult. On nonenhanced MRI, the nidus is best visualized on T2 weighted imaging (T2‐WI) sequences where it appears as a hypointense lesion surrounded by bone marrow edema. The nidus is a round lesion of intermediate signal intensity on T1 weighted imaging (T1‐WI); it may occasionally be seen as a focal signal void due to matrix mineralization. Enhanced and dynamic series obtained after gadolinium application have been maintained to improve nidus detection [90]. Accompanying bone marrow edema, soft tissue mass, and presence of joint effusion can overshadow the typical features of the lesion, therefore MRI is less useful than CT for the evaluation of osteoid osteoma [69]. However, MRI is more sensitive than CT scan for detection of reactive changes in the soft tissue. Also, MRI is particularly useful in evaluating the effect of osteoid osteoma on the spinal canal and cord [69], as well as the extent of intra‐ and extra‐osseous reactive changes and the possible inflammation of adjacent soft tissues. CT is the modality of choice in diagnosis of osteoid osteoma, but in patients with atypical clinical presentations, in whom their pain does not respond to nonsteroidal anti‐inflammatory drugs or where no obvious abnormality on plain roentgenograms is reported, MRI is recommended to investigate the underlying cause. PET may also have a role in the diagnosis, treatment, and follow‐up of osteoid osteoma, although further evaluation is required to investigate its specific role and accuracy. Some authors suggest a role for PET specifically in spinal osteoid osteoma. A study has shown that tumor nidus manifests 18F‐FDG‐avid glucose metabolism, whereas the surrounding sclerosis does not [91]. In follow‐up cases of radiofrequency ablation (RFA), hypermetabolic activity is absent [92]. 68Ga PSMA PET/CT, which is used in prostate cancer staging and restaging, has been used to detect a case of osteoid osteoma. However, this modality requires more research work to prove its utility in the diagnosis and follow‐up [93]. SPECT/CT hybrid imaging using 99mTc‐labeled bisphosphonates or PET/CT with a tracer of 18F‐NaF demonstrates high radionuclide uptake along with anatomic delineation and can accurately provide diagnosis in osteoid osteoma. 18F‐FDG is not the proper radionuclide in osteoid osteoma. Although some osteoid osteomas may exhibit increased uptake of 18F‐FDG, this tumor typically shows no uptake [94] (Figure 22.2). Figure 22.2 Axial (a), coronal (b), and sagittal (c) CT images of an osteoid osteoma patient showing a hypodense osteolytic area in the left posterior of the third cervical vertebral body (a–c, arrows) accompanying a central nidus and sclerotic rim, typical for osteoid osteoma. Axial T2‐weighted (d) and sagittal STIR (e) MRI images of the cervical spine in the same patient showing hyperintense bone marrow edema in adjacent bone, extending to the posterior arc (d and e, arrows). Corresponding planar bone scintigraphy images (f) show avid radiotracer uptake in the upper cervical spine (arrows) due to increased bone turnover. Osteoblastoma is an uncommon primary benign bone neoplasm with locally aggressive behavior [95]. Jaffe and Lichtenstein described it as a distinct entity in 1956 [96]. Osteoblastoma is commonly located in the spine, occurs in the second or third decade of life [97], and is more common in men [75]. Thoracic, lumbar, and cervical regions are equally affected. It affects the neural arch, especially lamina and pedicles, and may present with neurologic symptoms secondary to the compression of nerve roots [98] or pathologic fracture. Osteoblastoma is in differential diagnosis with osteoid osteoma [99]. However, osteoblastoma is larger (>2 cm), tends to be more aggressive, and may rarely undergo malignant transformation, while osteoid osteoma is benign, small, and self‐limited with no malignant potential [100]. Primary symptoms in osteoblastoma are pain, paravertebral muscular spasm, and stiffness. Unlike the pain in osteoid osteoma, the pain of osteoblastoma is dull, more generalized, and does not usually relieve by salicylates [101]. Scoliosis may appear in osteoblastoma. Osteoblastoma and osteoid osteoma can be difficult to differentiate on imaging. On plain X‐ray, osteoblastoma appears as a lucent, expansile, and well‐defined lesion of low density, with cortical thinning or rupture and a distinct eggshell calcification around the focus; however, due to osteoid and bone components, varying degrees of sclerosis may be seen [102]. In the aggressive type, cortical bone destructions and epidural or paravertebral bone expansion may also be seen [103]. CT scan provides detailed information about the extent, location, size, and borders of the tumor. It is the best modality for observation of the fine structure of the tumor and the formation of a bone shell, as well as evaluating soft tissue masses. CT can also show cortical bone destruction in aggressive cases. CT is better than X‐ray in showing osteolytic lesions and trabecular or bone mineralization. Sometimes multiple foci of matrix mineralization or extensive sclerosis may be observed. Cortical expansion is sometimes demarcated by a thin shell of bone. CT scanning before surgery has been demonstrated to be helpful for defining the exact location of the tumor and the extent of osseous involvement [84]. The imaging features on MRI are varied and generally nonspecific, and depend on the degree of tumor mineralization. On T1‐weighted images, the lesion is isointense or hypointense with a mixed hypo‐ and hyperintense signal on T2‐weighted images. Gadolinium‐enhanced MRI displays a rich vascularization in the osteoid tissue and reveals intense enhancement. Extensive reactive changes in adjacent vertebrae and neighboring soft tissue masses also enhance after contrast administration and may lead to overestimation of lesion size by MRI. CT scan should therefore still be the investigation of choice for the characterization and local staging of suspected spinal osteoblastomas. CT scan is also superior to MRI in showing scattered or extensive foci of calcification or ossification. MRI is the best method to reveal the effects of a tumor on the spinal canal and cord, as well as the extensive intra‐ and extra‐osseous reactive changes and the possible infiltration of adjacent soft tissues. 99mTc‐methylene diphosphonate (99mTc‐MDP) bone scanning is commonly used in osteoblastoma diagnosis because of active bone formation, especially for those with ambiguous or negative radiographic results. A 99mTc scintigram usually shows an increased uptake in osteoblastoma [104]. It is the most accurate means for localizing the tumor [98]. In spite of the benign characteristics of osteoblastoma, intense glucose metabolism of the tumor on 18F‐FDG PET/CT can suggest underlying malignant transformation [105] (Figure 22.3). Figure 22.3 MRI (a, b) and SPECT (c, d) of the thorax/cervical spine of a 16‐year‐old male with a history of surgical resection of osteoblastoma involving the C6 vertebral body during childhood. T1‐weighted (a) and T2‐weighted (b) axial MRI images at the level of C6 demonstrate a T1 isointense and T2 hypointense mass centered at the left neuroforamen. SPECT images of the thorax/cervical spine (c, d) demonstrate a metabolically active lesion centered on the left aspect of the C6. These features are compatible with local recurrence of the osteoblastoma. The patient subsequently underwent resection and spinal fusion. GCT is a benign lesion with aggressive propensity. It is sometimes difficult to distinguish it from malignancies both in anatomic imaging and with physiologic modalities. Around 7% of GCTs are found in the vertebral column, 90% of which are centered in the sacrum, particularly the ala [106]. In anatomic imaging, the presence of cortical destruction, endosteal scalloping, and sometimes soft tissue mass makes it difficult to distinguish it from malignant etiologies. GCT of the spine presents as a cystic, expansile lesion on plain radiograph. The lesion may lead to collapse of the vertebral body, ranging from mild collapse to a complete vertebra plana. Contrary to ABC, GCT usually affects the vertebral body. Some bony septa can arise from the border of the lesion extending to the inside of the mass, which is best seen in axial slices of CT. These bony septa may give a typical radiographic “soap bubble” appearance. Soft tissue outside the cyst is often seen on CT and MR scans and can suggest local aggression. This soft tissue could be misinterpreted as infection and is usually not seen in ABCs. Spine GCTs often show as heterogeneous signal intensity on all MR sequences. Generally, the solid components of GCT demonstrate low‐to‐intermediate signal intensity on T2‐weighted MR images owing to high collagen content as well as haemosiderin deposition. Although this feature is not unique to GCT of the spine, it is very helpful in narrowing the differential diagnosis because most of the other spinal neoplasms, such as metastases, lymphoma, and chordoma usually demonstrate a hyperintense signal on T2‐weighted images. Curvilinear low signal areas on both T1‐ and T2‐weighted images are other common MRI features of spine GCT, correlating with bony septae on CT. GCTs are also PET avid, showing high 18F‐FDG uptake with SUVmax of 4–5, limiting the role of 18F‐FDG PET/CT in distinguishing them from malignancy [55]. Even though this lesion is generally lytic, there are some reports demonstrating 18F‐NaF avidity [107]. This needs to be clarified with further studies. ABC is another lytic bony lesion and represents a blood‐filled cavity inside the bone. In the spine it primarily exists in the posterior elements of the vertebral column and it is often expansile with associated cortical thinning. The vertebral column can be affected in up to 20% of cases [108]. ABC tumors sometimes accompany other primary lesions of the bone, such as GCT, chondroblastoma, and osteoblastoma [109]. Plain radiographs show an expansile osteolytic cavity (ballooning of the vertebra). However, plain radiographs alone do not suffice in fully characterizing the lesion, necessitating cross‐sectional imaging modalities. Multiple fluid–fluid levels within the hemorrhagic cavities is a characteristic finding on CT and MRI. The presence of typical fluid–fluid levels on MRI is a characteristic feature of ABC. This appearance is due to the presence of blood in different stages of evolution within the cyst. On MRI, ABC can have a similar anatomic appearance as telangiectatic osteosarcoma, which is an aggressive malignancy. These two are often slightly hypermetabolic and cannot be differentiated reliably on 18F‐FDG PET. ABCs typically have SUVmax of 1–6 [55]. EG is another lytic lesion mostly found in children and usually occurring before age 30 [110]. Vertebral involvement can be variable, ranging from an isolated to often multiple lytic lesions, and can lead to partial or complete vertebral body collapse (vertebra plana), peridural spread, and soft tissue expansion around the spine. Vertebra plana can also be seen in other diseases, such as lymphoma, Ewing’s sarcoma, and infections such tuberculosis; however, EG is the most common cause of this appearance. Plain radiography is of limited value in the diagnosis of EG in the spine, and CT scan better defines the extent of the disease. On CT, the lesions are usually seen as punched‐out osteolytic radiolucent defects without reactive sclerosis or periosteal thickening. EG on MRI presents with low to intermediate signal intensity on T1‐weighting images and high signal intensity on T2‐weighting images, with marked enhancement. MRI is also useful in delineation of bone marrow involvement, any accompanying soft tissue mass or inflammation, and to assist with planning a biopsy or surgical excision. Due to high histiocytic content, lesions are usually 18F‐FDG avid, so 18F‐FDG PET is of little utility in differentiating EG from malignancy. 18F‐FDG PET is as sensitive as MRI in lesion detection and is often used as the modality of choice for screening and follow‐up in multifocal disease [111]. EG can also demonstrate substantial uptake of 18F‐NaF, despite being lytic [111]. The anatomic appearance in fibrous dysplasia is usually more characteristic of a benign lesion compared to more aggressive appearing lesions, such as GCT and osteoblastoma. Sometimes a more aggressive appearance, such as secondary ABC formation and cortical breakthrough, can be seen on CT [112]. There is often a classic ground‐glass appearance in the osseous matrix, with many lesions being expansile and demonstrating lytic components and a sclerotic rim on CT and X‐ray [3]. Although fibrous dysplasia has a mean SUVmax of approximately 2, many lesions exhibit a much higher SUV, making them difficult to differentiate from malignant lesions, such as metastatic disease, chondrosarcoma or osteosarcoma, based on 18F‐FDG avidity [113]. Vertebral involvement of fibrous dysplasia is rare, but can be seen with polyostotic forms. Due to osteoblastic activity, fibrous dysplasia is also 18F‐NaF avid, further confounding it with more aggressive tumors on the basis of physiologic imaging alone [114] (Figure 22.4). Figure 22.4 CT images (a, b), bone scan (c), and PET‐CT (d) images of a 38‐year‐old male with a neck mass and pathologically proved fibrous dysplasia. Axial (a) and coronal (b) CT images demonstrate an expansile osseous lesion centered within the right vertebral rib. Increased radiotracer uptake on both bone scan (c) and FDG PET/CT (d) is noted. These features are consistent with monostotic fibrous dysplasia. As these lesions can remain metabolically active into adulthood in patients with malignancy, it is important not to mistake this for a metastatic lesion. Hemangioma is the most common benign lesion of the spine, found frequently in middle‐aged individuals [73]. Although asymptomatic, some lesions can become expansile and compress the spinal cord [115]. They are often simple to diagnose on anatomic imaging modalities, such as CT and MRI, exhibiting a classic corduroy appearance of vertical trabeculations, with portions of fat equivalent signal on MR imaging. Expansile hemangiomas can appear aggressive, sometimes being confused with Ewing sarcoma or other vascular lesions, such as hemangioblastoma. Hemangiomas are classically not 18F‐FDG avid, with SUVmax less than 2, therefore 18F‐FDG PET can help distinguish these lesions from other malignant lesions [57]. However, case reports of 18F‐FDG‐avid lesions have been described [116]. On 18F‐NaF PET, hemangiomas often exhibit significant radiotracer uptake [114] (Figure 22.5). Aside from metastatic bone disease, hematologic malignancies, such as MM and lymphoma, are the most common malignant spinal lesions. MM is the most common primary bone malignancy in adults. Solitary plasmacytoma is one of the most common primary osseous lesions of the pelvis and vertebrae [117]. 18F‐FDG PET/CT has shown advantage over 18F‐FDG PET alone in detecting lesions less than 10 mm and in distinguishing diffuse spine involvement of MM [118]. In a study comparing 18F‐FDG PET/CT and technetium sestamibi (99mTc‐MIBI), 99mTc‐MIBI better detected multifocal disease [119], while PET/CT better detected focal lesions [120]. 18F‐FDG PET/CT has also been shown to be twice as sensitive as 18F‐NaF PET/CT in detecting myeloma lesions [121]. Although 18F‐FDG PET/ CT has good sensitivity for sizeable MM lesions, PET/MR imaging has shown a greater sensitivity for small focal lesions and can be a promising modality that can help better define neural compromise and assess the extent of lesions. Figure 22.5 Sagittal CT scan (a) and PET/CT (b) images of a 61‐year‐old male with a history of bladder cancer. On CT, there is typically a trabeculated appearance of hemangioma in the L3 vertebral body and no uptake in the FDG PET/CT. Hemangiomas typically do not demonstrate increased radiotracer uptake on FDG‐PET. Primary lymphoma of the spine is a rare form of extranodal lymphoma. This entity constitutes a diagnostic challenge due to its mimicking of other spinal diseases and the difficulty in establishing a tissue diagnosis. Primary bone lymphoma is less common than secondary involvement of bone from systemic lymphoma. These are more commonly non‐Hodgkin histologic subtypes, and only 6% of diagnosed cases have been Hodgkin lymphoma. When facing a patient with a suspected lymphoma of the spine, it is first important to rule out other sites of involvement with imaging, including PET/CT, in order to differentiate between primary spinal lymphoma and a secondary metastatic spread of a systemic lymphoma. The initial workup is similar to that of nodal lymphoma. Primary lymphoma presents on MRI as iso‐ or hypo‐intense homogenous tumor with enhancement, involving multiple segments. The absence of hyperintensity, although not a consistent finding, helps in differentiating it from metastasis or hematomas. Primary bone lymphoma are extremely 18F‐FDG avid, with SUV values up to 10 and even more compared to approximately 5 in other malignant primary bone lesions [121]. Up to 14% of primary bone lymphoma involving the spine can have epidural extension [122]. Since timely treatment can improve prognosis substantially in these patients, compared to other bone malignancies, early and proper diagnosis is consequential [123] (Figure 22.6). A chordoma is a low‐grade, slow‐growing, but locally aggressive tumor. Chordomas arise from the remnants of the notochord and occur in the midline along the spinal axis from the clivus to the sacrum, anterior to the spinal cord, and typically present in the fifth and sixth decades. Chordomas are 50% sacral, 35% base of skull, and 15% in the vertebral bodies of the mobile spine. CT scan is helpful in detecting any bone destruction and evaluating the status of cortical bone, periosteal reaction, and calcification. Usually the destruction has a mixed lytic‐sclerotic appearance that is similar to the appearance of a melting ice or sequestrum within osteomyelitis. Vertebral sclerosis can be a feature of chordoma and is thought to be due to reactive change of the trabecular architecture. Lytic bone destruction with retention of trabecular architecture is a feature of an aggressive yet slow‐growing variant, which can also be observed in vertebral hemangiomas. Chordoma is classically extremely T1 hypointense and T2 hyperintense on MRI. When an 18F‐FDG‐avid sacrococcygeal lesion is noted on PET/CT, the possibility of chordoma should also be considered [124]. Chordoma shows heterogeneous 18F‐FDG uptake and causes local bony destruction in the sacrum, often extending into the sacral epidural space, causing nerve root compromise and having a soft tissue component [125] (Figure 22.7). Figure 22.6 CT scan (a), MRI (b), and PET/CT (c) of a 39‐year‐old male with a history of HIV, hepatitis C, and Burkitt’s lymphoma. Midline sagittal CT demonstrates a slightly heterogenous appearance of the bone with compression fracture of the L5 vertebral body. The T1 post‐contrast fat‐saturated image reveals extensive infiltrative and enhancing bone marrow replacing disease. PET‐CT demonstrates diffusely increased metabolic activity throughout the lumbosacral spine. These features are compatible with bone marrow replacing lymphoproliferative disorder. The extent of the disease is better defined on PET‐CT and MRI than CT scan alone. Ewing sarcoma and osteosarcoma are the most common nonhematologic primary vertebral malignancies. Compared to osteosarcoma in the limbs, vertebral involvement usually happens in older age, with a mean of 38 years. It is more common in men. The tumor typically arises in posterior elements of the spine with partial involvement of the vertebral body. X‐ray and CT in osteosarcoma usually have a mixed osteosclerotic–osteolytic appearance. A heterogenous soft tissue mass with ossified and nonossified components usually accompanies the lesion. Ivory vertebra is a rare manifestation seen in sclerosing osteoblastic osteosarcoma. A purely lytic pattern may also be seen in some variants, such as telangiectatic osteosarcoma. Spinal osteosarcoma has nonspecific MRI findings. The mineralized components of the tumor are low signal on both T1‐ and T2‐weighted images, while nonmineralized parts exhibit high signals on T2. Fluid–fluid levels have been reported in telangiectatic osteosarcoma. Soft tissue extension, expansive pattern cortical destruction, and pathological fractures may also be seen. Ewing sarcoma has a peak incidence in the second decade of life with a mean age of 19 and a relative male predominance. Vertebral column is affected in 3–10% of cases, with the sacro‐coccygeal region being the most common site of involvement, followed by lumbar and thoracic spine. Like osteosarcoma, lesions usually originate in the posterior elements of the spine and extend into the vertebral body. Pain is the most common symptom. Radiculopathy and neurological deficit are seen in up to 80% of cases. Lesions can be lytic, sclerotic, or mixed. Most lesions are lytic and morphologically aggressive, with large paraspinal soft tissue components that are usually larger than the parent intraosseous lesion. Ivory vertebra, vertebra plana, and pseudohemangioma are other unusual imaging findings. Invasion of the spinal canal is very common and happens in up to 91% of cases.
22
Spine Disorders: Correlative Imaging Approach
Spinal Infection
Transmission Mode and Infection Predilection
Microbiology
Clinical Features
Role of Plain X‐rays
Role of CT Scans and MRI
Bone Scintigraphy, SPECT/CT, and PET/CT
MRI and PET/CT: A Correlative Approach in Diagnosis
Primary Spinal Tumors
Benign Spinal Lesions: A Correlative Approach in Diagnosis
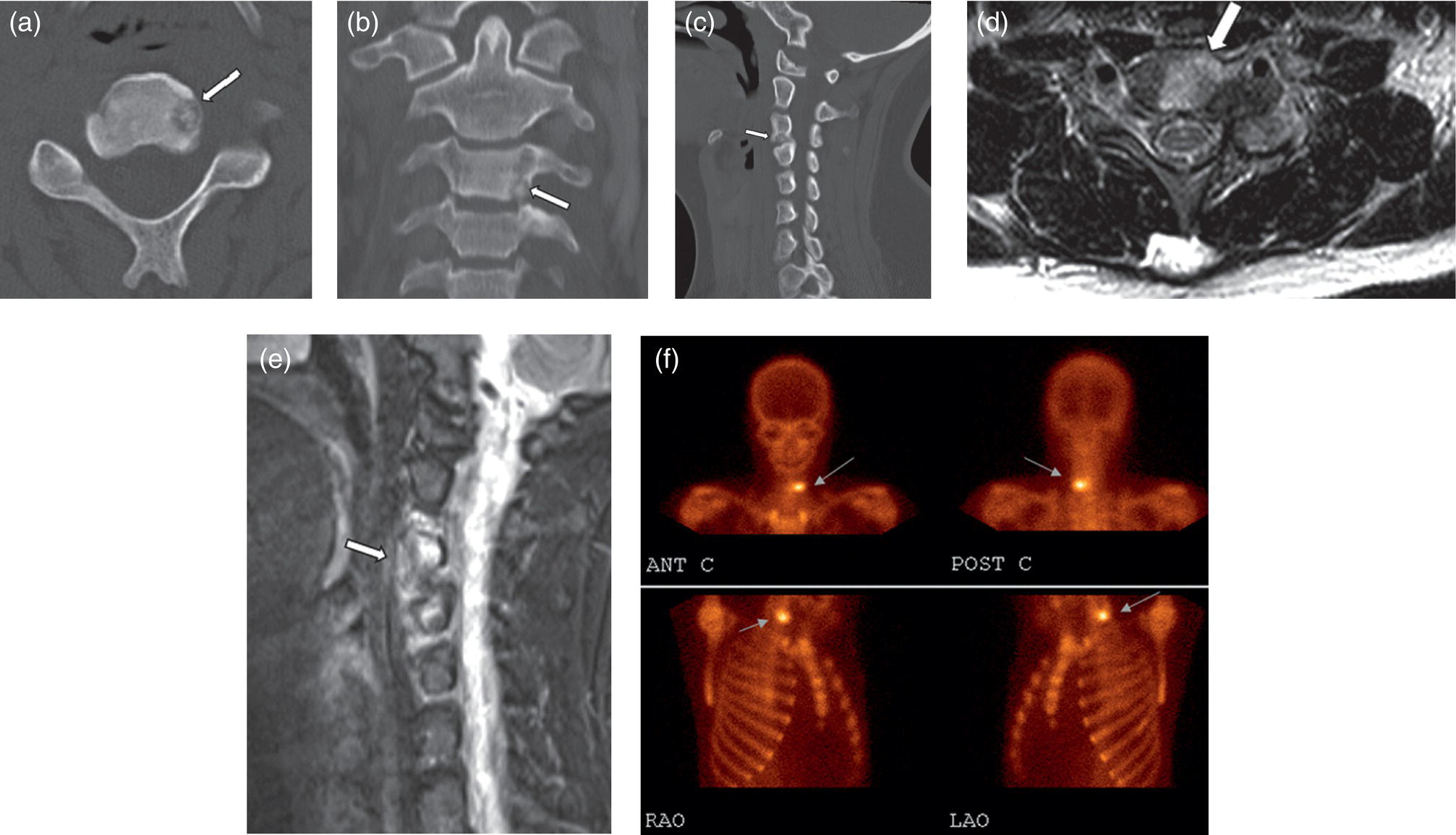
Osteoid Osteoma
Clinical Features
Role of Plain X‐ray
Role of CT scan
Role of Scintigraphy
Role of MRI
PET, SPECT/CT, and PET/CT: Correlative Approach
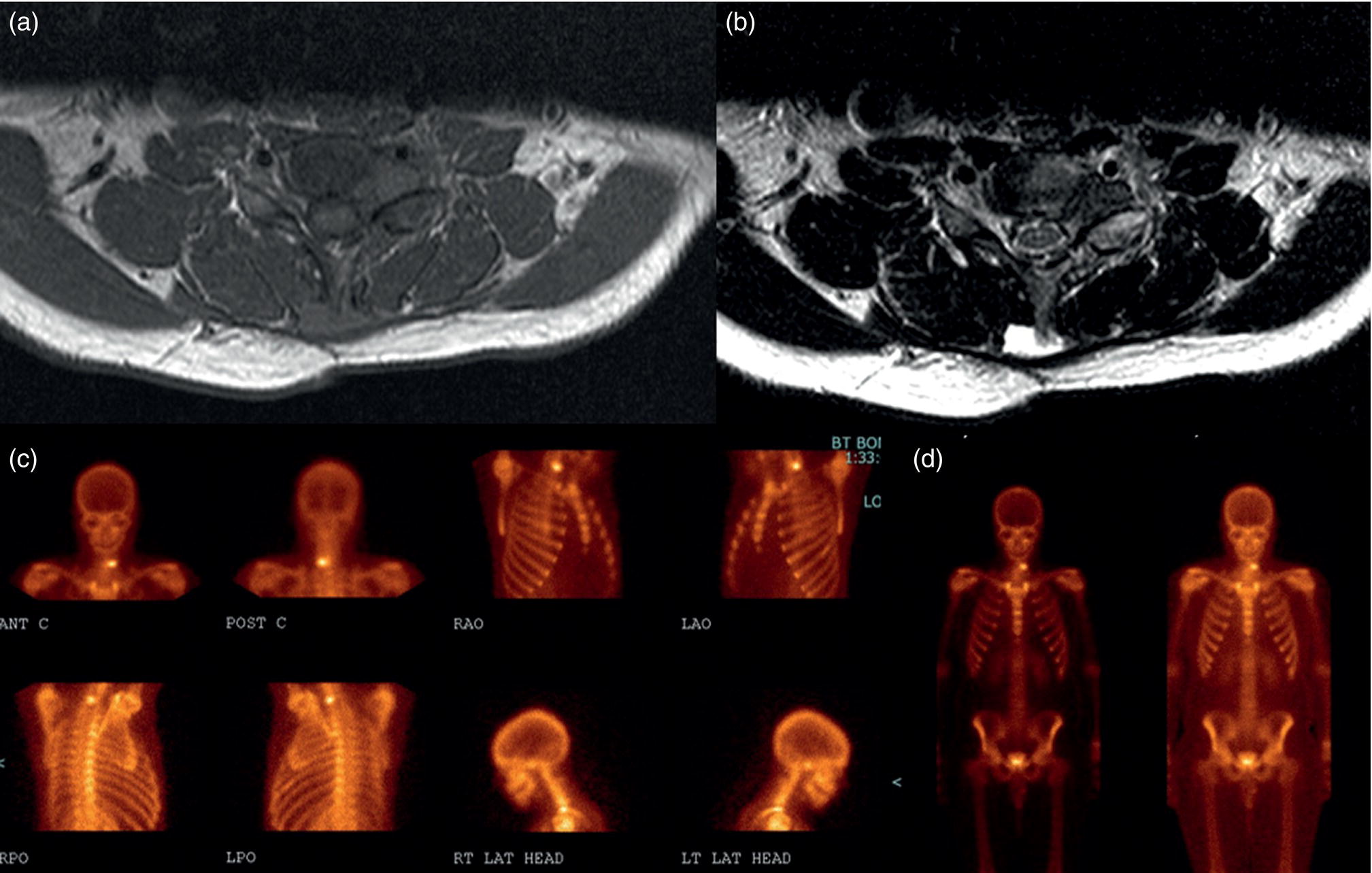
Osteoblastoma
Role of X‐ray
Role of CT Scan and MRI
Role of Bone Scan and PET/CT
Giant Cell Tumor
Aneurysmal Bone Cyst
Eosinophilic Granuloma
Fibrous Dysplasia
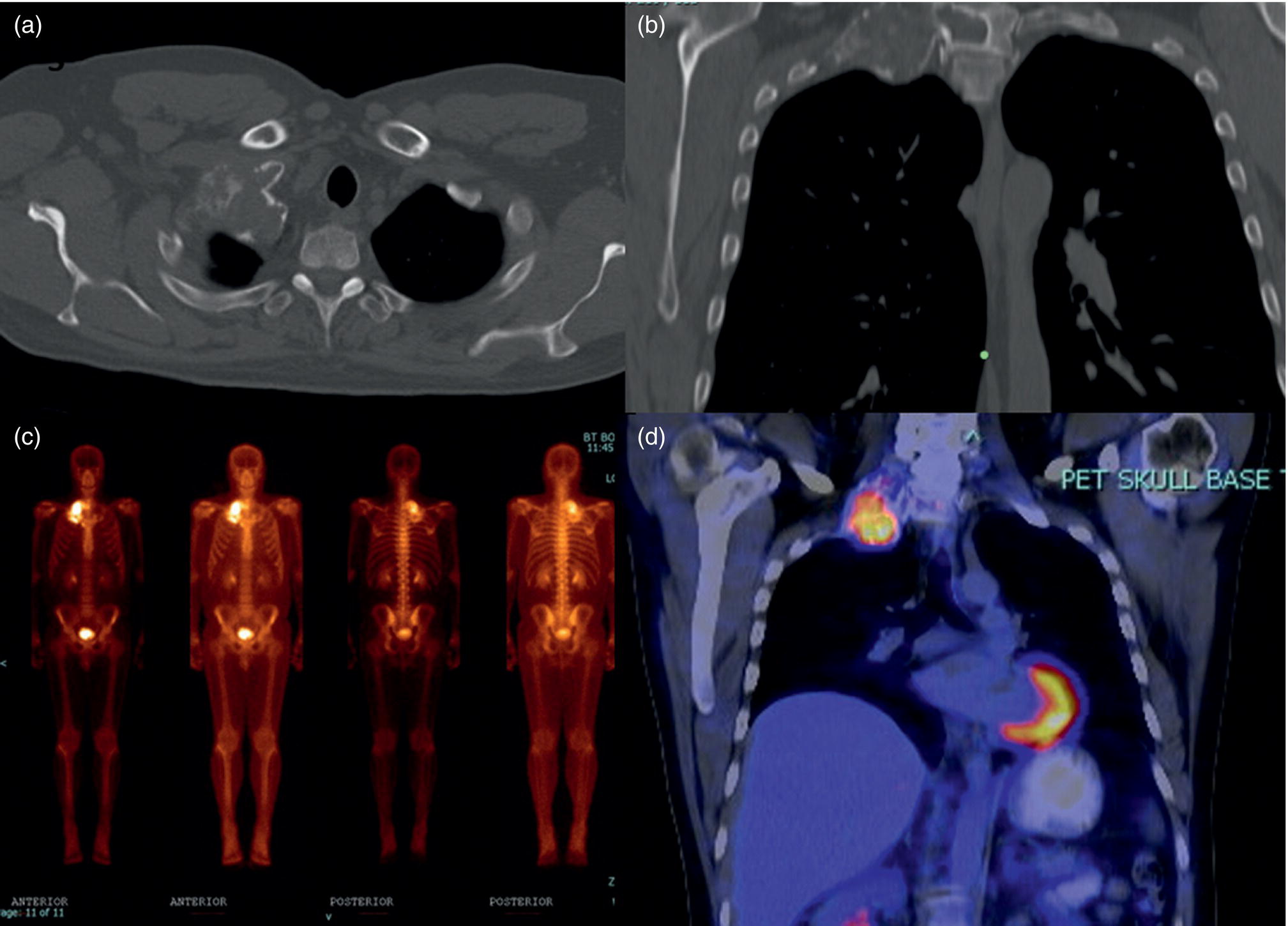
Hemangioma
Malignant Spinal Lesions
Correlative Approach in Diagnosis
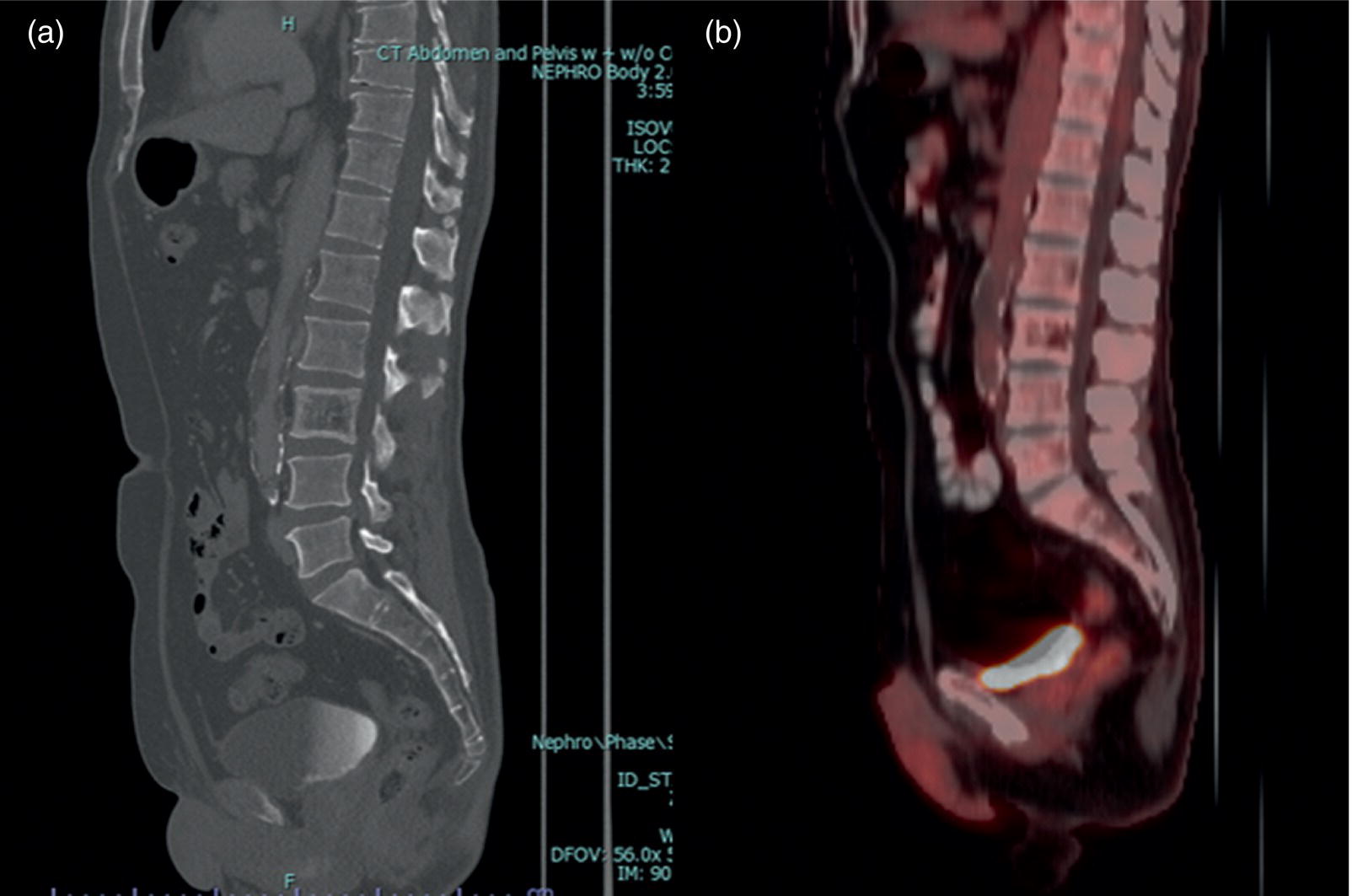
Lymphoma
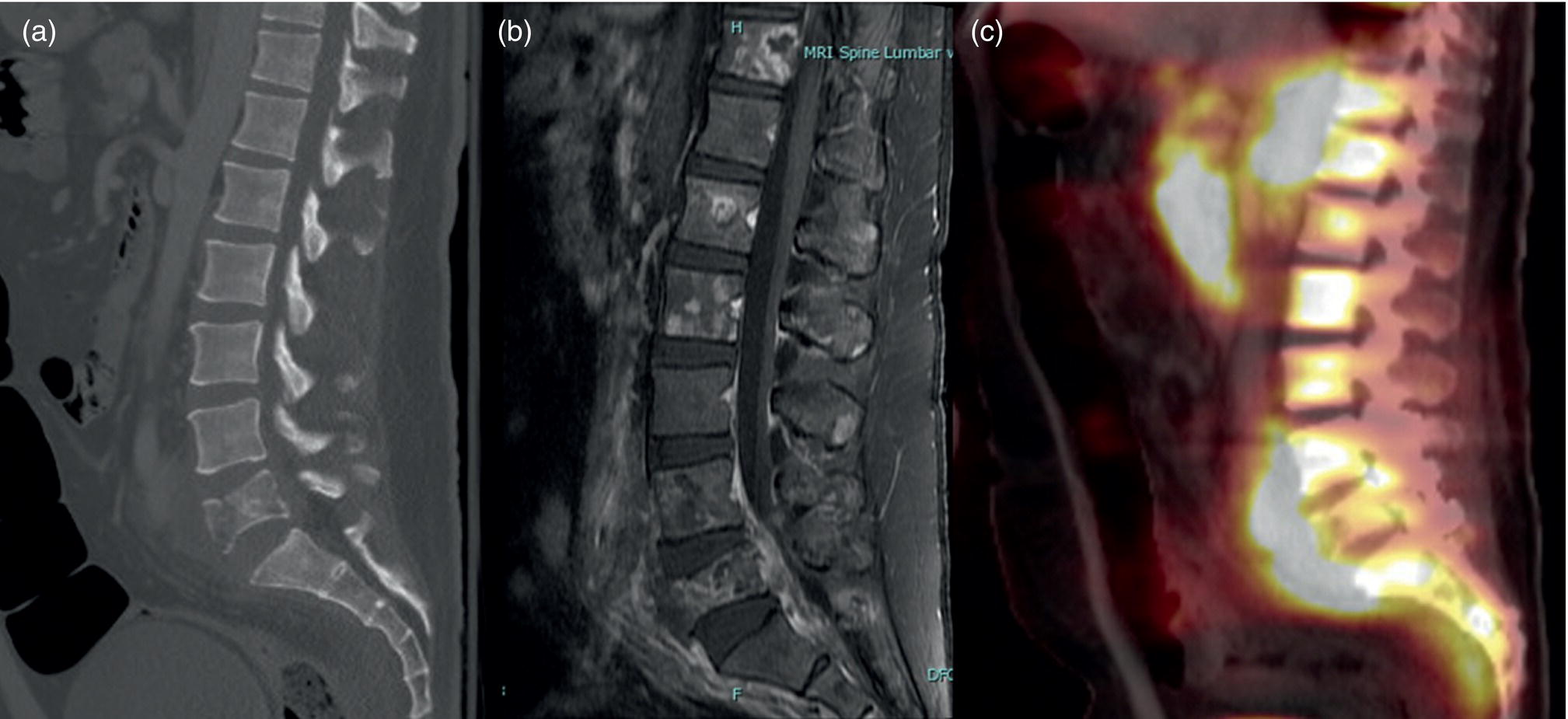
Chordoma
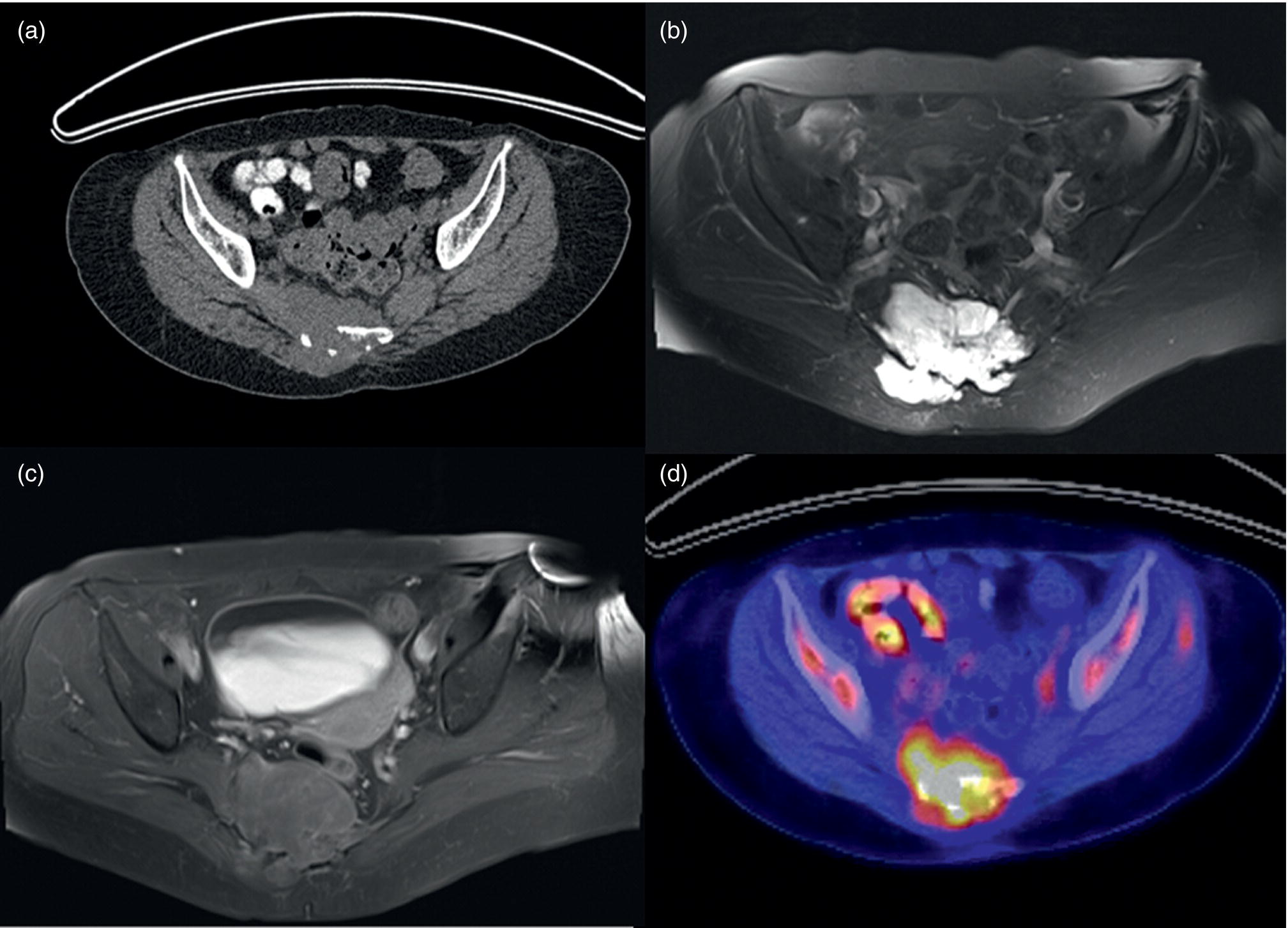
Ewing Sarcoma/Osteosarcoma

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree