Chapter 37 Stroke is an important cause of mortality and long-term neurologic morbidity in children. In the pediatric age group, it is defined as a cerebrovascular event occurring between 14 weeks of gestation and 18 years of life. It ranks among the top 10 causes of death in children,1 with the highest incidence observed in the perinatal period. It occurs in approximately 25 per 100,000 live births in the neonatal population and in 2 to 3 per 100,000 in children between 30 days and 18 years. Recurrence is estimated to be around 3% to 5% in neonates and ranges from 20% to 40% in older children. Among those who survive, more than 50% progress to the development of permanent neurologic or cognitive sequelae.2 The required treatment and rehabilitation programs usually result in a large economic burden to the family and society (Box 37-1). Maternal, placental, and fetal risk factors have been reported, but in more than 50% of cases, no obvious cause is found. The common maternal conditions associated with fetal stroke are alloimmune thrombocytopenia, diabetes, anticoagulant or antiepileptic therapy, and trauma. Placental factors include placental hemorrhage, abruption, and thromboemboli.3 It is unclear whether coagulopathy is a risk factor, but a case of fetal protein C deficiency has been reported. Once an abnormality is found on a prenatal ultrasound examination, fetal magnetic resonance imaging (MRI) usually is performed; it is the imaging modality of choice for assessing fetal brain injury (Fig. 37-1). Hemorrhagic lesions have been reported in more than 90% of cases, compared with porencephalic cysts, which are reported in 10% of cases. Arterial ischemic stroke (AIS) typically involves the major arterial territories, most commonly the middle cerebral artery (MCA). Arterial ischemic insults occurring in the second trimester can cause cortical disorganization, resulting in polymicrogyria. If fetal hemorrhagic strokes are similar in origin to the vast majority of preterm and term hemorrhages, it is likely that many fetal hemorrhagic strokes are venous strokes. When tissue destruction occurs as a result of a fetal stroke, the type of tissue response identified on postnatal imaging can help determine the time of the intrapartum event. Porencephalic cysts lack a surrounding astroglial response and develop with injuries between 22 and 27 weeks of gestation. Thereafter, cystic encephalomalacia with gliosis is seen on pathology and MRI. Unlike in neonates and adults, diffusion-weighted imaging (DWI) may not be reliable in predicting the approximate date of an event.4 Figure 37-1 Fetal stroke. Perinatal or neonatal stroke is an event that occurs between the late third trimester and the first month of life. The pathophysiology is complex and typically multifactorial. Recently, prothrombotic abnormalities of the coagulation pathway have been of particular interest because of the evolving role and potential use of antithrombotic agents for both treatment and prevention.5 Perinatal AIS leads to focal ischemic necrosis in an arterial distribution, most commonly in the MCA territory. The cause is undetermined in more than half of all cases. In the remainder of cases, the source of the thromboemboli may be an intracranial or extracranial vessel, the heart, or the placenta. An increased incidence of dehydration and sepsis also is found, along with cardiac and coagulation disorders.2 AIS may be clinically subtle, and newborns often present with seizures without encephalopathy 2 to 3 days after birth. At the time of clinical presentation, ultrasound of the head can be have false-negative results. Computed tomography (CT) can detect hemorrhage and areas of advanced infarction but also may have false-negative results. Furthermore, ionizing radiation exposure is discouraged in neonates. Acute AIS is easily identified on MRI as regions of bright signal on DWI and decreased signal on apparent diffusion coefficient (ADC) maps within a vascular territory. The reduction in ADC values results from the presence of acute ischemic necrosis and the associated physiologic changes, such as cellular swelling, increased tortuosity of the extracellular space, decreased intracellular cytosolic streaming, and increased intracellular viscosity. The reduction in ADC can persist for up to 2 weeks, being more conspicuous during the first 4 days.6 On T2-weighted images, subtle loss of gray-white matter differentiation often is identified, although it may be negative within the first hours after clinical presentation. MR angiography (MRA) may be helpful in excluding complete occlusion of a major intracranial artery, but turbulent or fast flow often can result in signal dropout, which generates a concern for partially occlusive thrombus in this clinical context. Cerebral perfusion can be obtained using arterial spin labeling. This technique uses arterial blood water magnetically labeled by a radiofrequency pulse to obtain cerebral blood flow measurements; it does not require intravenous injection of contrast media (see Chapter 28). This technique can be particularly useful in determining the presence of reperfusion in areas of abnormal ADC (Fig. 37-2).7 Figure 37-2 Neonatal stroke.
Stroke
Fetal Stroke
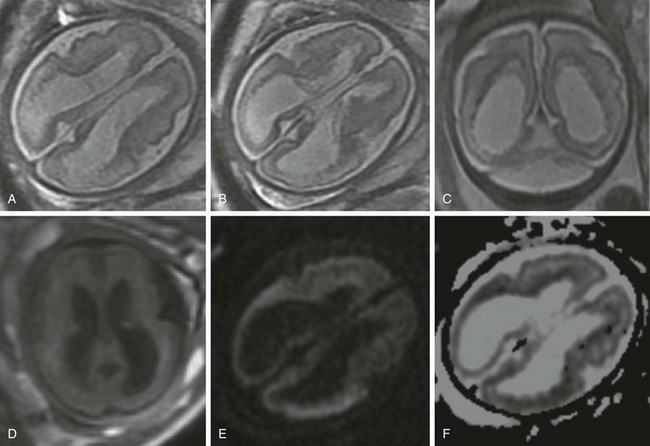
A 30-year-old woman underwent a magnetic resonance imaging scan after fetal ventriculomegaly was found on a routine prenatal ultrasound at 29 weeks’ gestational age. A, B, and C, T2-weighted images show dilatation of the lateral ventricles associated with T2 prolongation, thinning, and irregularity of the periatrial white matter. D, T1-weighted image. E, Diffusion-weighted image. F, Apparent diffusion coefficient map. Reduced diffusion also is observed within the periventricular white matter, suggesting evolving ischemic necrosis.
Perinatal or Neonatal Stroke
Arterial Ischemic Stroke
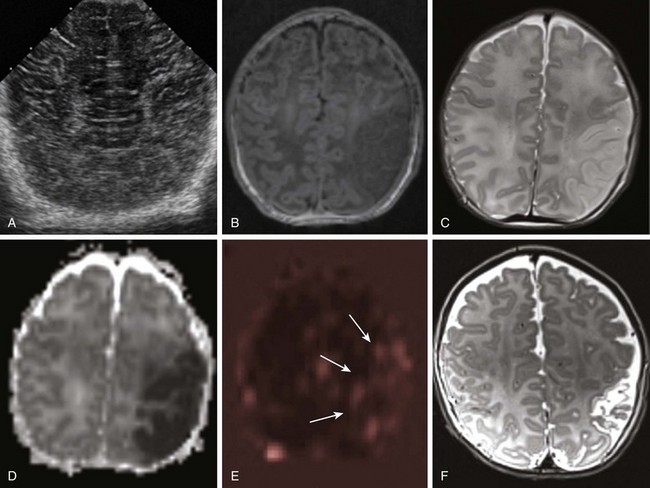
A 1-day-old full-term infant with seizures. A, Ultrasound of the head shows normal cerebral architecture. A magnetic resonance imaging scan performed 10 hours later shows edema (B, T1-weighted image; C, T2-weighted image) and decreased diffusion (D, apparent diffusion coefficient map) involving gray and white matter in the left middle cerebral artery (MCA) territory. Perfusion-weighted images demonstrate relative increased blood flow in the same region (arrows in E, arterial spin labeling). A follow-up T2-weighted image at 3 months (F) shows encephalomalacic changes and volume loss within the area of the prior MCA territory infarct.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stroke