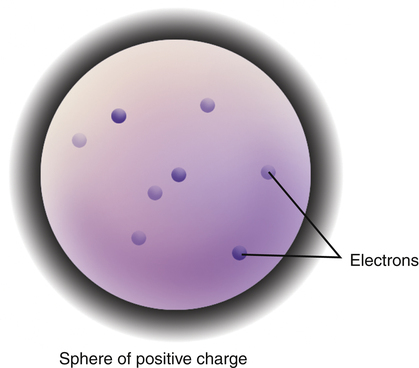
• Describe the nature and structure of the atom. • Identify the constituents of the atom and the characteristics of each. • Explain classifications of the atom. • First, the interactions in the x-ray tube that produce x-rays occur at the atomic level, and the nature of the x-ray photon produced depends on how an electron interacts with an atom. • Second, the interactions between the x-ray photons and the human body also occur at the atomic level, determining both the radiation dose delivered and how the body part will be imaged. • Third, the interactions between the x-ray photons exiting the patient to produce the image interact at the atomic level of the image receptor to generate the final image. • Finally, other areas of study in the radiologic sciences also require a working knowledge of the atom. So it is best to develop a strong foundation at the outset. Although it is believed that some basic ideas of atomism or atomic theory predate Leucippus, his name most often is associated with the earliest atomic theory. His ideas were rather vague, and it is his student and follower, Democritus of Abdera, who provided one of the most detailed and elaborate theories and is credited with expanding on and formalizing the earliest atomic theory. Democritus lived from about 460 BC to about 370 BC. The name atom comes from the Greek word atomos, meaning “indivisible.” Democritus hypothesized that all things were made of tiny, indivisible structures called atoms. Figure 2-1 illustrates early Greek theory of atoms. Democritus believed that these atoms were indestructible and differed in their size, shape, and structure. He theorized that the nature of the object depended on its atoms. For example, sweet things are made of smooth atoms and bitter things of sharp atoms. Solids consist of small pointy atoms, liquids of large round atoms, and so on. Such ideas and theories were debated and carried forward for another 2000 years. The English chemist John Dalton in the early 1800s developed a sound atomic theory based not on philosophical speculation but on scientific evidence. His recognition that elements combined in definite proportions to form compounds led to questions about why this happened. This inquiry led in turn to his atomic theory. Figure 2-2 is a photo of Dalton’s original wooden models of the atom. To explain the phenomenon, he theorized that all elements were composed of tiny indivisible and indestructible particles called atoms. These atoms were unique to each element in their size and mass. From this he theorized that compounds were formed by molecules and molecules by fixed ratios of each type of constituent atom, resulting in a predictable mass. Finally, his theory stated that a chemical reaction was a rearrangement of atoms. His theory is now more than 200 years old but remains fundamentally valid. We know now that we can destroy the atom in a nuclear reaction, but his basic ideas were correct. Later Dmitri Mendeleev advanced Dalton’s work by organizing the known elements into the periodic table, which demonstrates that elements, arranged in order of increasing atomic mass, have similar chemical properties. The next significant advancement in atomic theory came with Joseph John “J.J.” Thomson’s discovery of the electron. This discovery resulted from the scientific community’s fascination with the cathode ray tube, the very fascination that led Dr. Roentgen to his discovery of x-rays. Thomson was studying the well-known glowing stream that is visible when an electric current is passed through the cathode ray tube. This glowing stream was familiar to scientists, but no one knew what it was. Thomson discovered that the glowing stream was attracted to a positively charged electrode. Through his investigation of this phenomenon, he theorized that these glowing particles were actually negatively charged pieces of atoms (later named electrons). Based on his understanding, he described the atom as a positively charged sphere with negatively charged electrons embedded in it, much like the raisins in a plum pudding—hence its name: the “plum pudding model.” See Figure 2-3. Thomson’s theory was further advanced by one of his students, Ernest Rutherford. Marie and Pierre Curie had recently discovered radioactivity, and Henri Becquerel, radioactive rays. Rutherford was conducting scattering experiments by bombarding a very thin sheet of gold with alpha particles. Alpha particles are made up of two protons and two neutrons (basically the nucleus of a helium atom) and have a positive charge. He placed a zinc sulfide screen in a ring around the gold sheet and observed the experiment with a movable microscope (Figure 2-4). He observed that most particles passed straight through the sheet, but some were deflected at varying angles from slight to 180 degrees back along the path they had traveled. To Rutherford, this suggested that there were tiny spaces, or holes, at the atomic level. This space allowed most of the particles to pass through, but some particles hit parts of the atoms. Such an idea contradicted his teacher’s model and, based on his experiments, he proposed a new, rather different model of the atom. His model resembled a tiny version of our solar system. He described a positively charged and very dense nucleus with tiny electrons orbiting it in defined paths. This model explained how some of the alpha particles could pass right through the gold sheet (between the nuclei of the atoms and missing the orbiting electrons) whereas others were deflected (repelled by the strong, positively charged nucleus). His version was a radically new idea, but it did not explain a couple of physical principles of nature. The 20th-century Danish physicist Niels Bohr refined Rutherford’s work, bringing us to the theory and model of the atom with which we are most familiar.
Structure of the Atom
Introduction
Basic Atomic Structure
Historical Overview

Dalton’s wooden models of the atom.
Reprinted with permission Science Museum (London).
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine