1 The Basics of IR Being a trainee in IR can be intimidating. The specialty sees a wide variety of pathology, and performs over a hundred different procedures. The equipment and imaging techniques used require some time to get acclimated to. All of this can be overwhelming. However, with some fundamental knowledge and the tips outlined in this book, you can hit the ground running on your first IR rotation. When it comes to learning IR procedures, there’s no substitute for getting your feet wet and participating in cases. While it is important to get as much procedural experience as you can, you’ll also need to be attentive to the clinical responsibilities expected of you as a trainee on the service. This includes answering the phone, fielding consults, and interfacing with other clinical teams. You should take full ownership of patients scheduled for procedures during the day, postprocedure patients, and the new consults as they come in. Your day starts with reviewing the scheduled cases. This includes reviewing the indication for the procedure, any relevant imaging available, and any pertinent progress notes. You’ll learn quickly that IR is a fast-paced specialty. Preparing ahead of time will make the day run much smoother. Consults for IR can come at any time; some are urgent or emergent, while others may be routine. A good habit to get into is to follow the same set of steps for each new consult so that you don’t overlook any important details (▸Table 1.1). Determine the nature of the clinical problem and the expectations of the referring service. There should be a conversation where you gather from the referring team the underlying clinical problem, the interventions that have been performed thus far, and the urgency. You’ll need to make sure you understand what they are hoping to gain from the IR procedure. If the consult is for a diagnostic procedure, what information is being sought? Good communication will ensure that both the IR team and the referring service have an understanding of what will be done. If an urgent consult is requested, gather the bare bones information (clinical history, hemodynamic status, laboratory values, imaging), but don’t delay in notifying your attending. He or she may decide to bring the patient straight to IR. If the consult is nonurgent, you have time to perform a more thorough work-up before staffing the case. An ideal presentation will include the clinical history, imaging findings, your final assessment, and a proposed plan of action.
1.1 The IR Consult
What Is the Reason for the Consult?
Does the Patient Need to Be Seen Immediately?
What is the reason for the consult? |
Does the patient need to be seen immediately? |
Are there alternatives to IR treatment that are more appropriate, and have they already been attempted? |
Is the procedure technically feasible? |
Are there any safety concerns that need to be addressed? |
Are There Alternatives to IR Treatment That Are More Appropriate, and Have They Already Been Attempted?
It is important to have some foundational knowledge of the diseases commonly treated by IR, and how the IR procedure fits into the bigger picture. Unfortunately, this is not always cut and dry. Many IR procedures do not fall neatly into an algorithm. A good understanding of the clinical context is required to determine if and when IR should get involved.
Is the Procedure Technically Feasible?
If an IR intervention is potentially indicated, review the relevant imaging available to you. As radiologists, our expertise in imaging interpretation allows us to learn a great deal about the patient before we even meet them. We can plan out a procedural approach, identify anatomic variants, and look for potential pitfalls. As a trainee, you may not have a strong foundation in imaging, so it’s okay to ask your senior residents or an attending for help.
Are There Any Safety Concerns That Need to Be Addressed?
If the conditions above are met, the next step is completing a basic preprocedure work-up. This can be time consuming, but it is important to review before officially approving a procedure. This is part of what separates clinicians from technicians.
Bleeding Risk
Bleeding risk should be assessed for any patient undergoing an interventional procedure. Society of Interventional Radiology (SIR) guidelines stratify procedures into low, moderate, and high risk of bleeding. Low-risk procedures are those which are either minimally traumatic with small access devices, or those in which access is obtained into a space in which bleeding would be easily detected and controlled. Examples include paracentesis, drainage catheter exchange, superficial biopsies, peripherally inserted central catheter (PICC) placement, and IVC filter placement. Moderate-risk procedures include the majority of interventional procedures, including most arterial and venous interventions, and tunneled line placement. High-risk procedures are those in which a solid organ is traversed, including transjugular intrahepatic portosystemic shunt (TIPS), biliary interventions, and nephrostomy access.
The two laboratory tests that are most important in determining the bleeding risk are platelets and international normalized ratio (INR). For all procedures, platelets need to be greater than 50,000. For low-risk procedures, the INR needs to be below 2. For moderate- and high-risk procedures, the INR needs to be below 1.5 (▸Table 1.2).
The number of patients on long-term anticoagulation has increased in recent years, and there are now a wide variety of medications used for this purpose (see Chapter 9). The preprocedural assessment should look into any history of bleeding or clotting disorders, as well as review use of warfarin, heparin, antiplatelet agents, or the newer factor Xa or direct thrombin inhibitors. Screening laboratory tests should include INR, partial thromboplastin time (PTT), and platelets (+/− anti-Xa levels). Note that PTT/INR takes into account only a portion of the overall clotting cascade.
For those patients on anticoagulation, reversal agents may be necessary in order to expedite an IR procedure. These include protamine sulfate for heparin, idarucizumab for dabigatran, and vitamin K for warfarin. In some cases, reversal is accomplished with the use of blood products. Options include fresh frozen plasma (FFP), prothrombin complex concentrate (PCC), platelets, and cryoprecipitate (▸Table 1.3).
Table 1.2 IR procedure risk stratification based on the bleeding risk
Risk category | Procedures | Laboratory thresholds |
Low risk | PICC insertions, dialysis interventions, IVC filters, thora-/paracentesis, superficial biopsies | INR < 2 |
Moderate risk | Any procedure requiring an arterial stick and intervention up to 7 Fr, venous interventions, embolizations, tunneled central catheter, port placements, perc chole, liver biopsy, abscess drainage, lung biopsy, spine procedures | INR < 1.5 |
High risk | TIPS, PTC/PTBD, percutaneous renal procedures (nephrostomy tube, biopsy) | INR < 1.5 |
Abbreviations: perc chole, percutaneous cholecystostomy; PICC, peripherally inserted central catheter; PTBD, percutaneous transhepatic biliary drainage; PTC, percutaneous transhepatic cholangiography; TIPS, transjugular intrahepatic portosystemic shunt. | ||
Table 1.3 Reversal agents for common anticoagulants
Anticoagulant | Reversal agent |
Warfarin | Immediate reversal: FFP or PCC + vitamin K Semiurgent reversal: vitamin K |
Heparin (including enoxaparin/LMWH) | Protamine sulfate |
Dabigatran | Idarucizumab |
All other novel oral anticoagulants | PPC; new reversal agents being studied |
Abbreviations: FFP, fresh frozen plasma; LMWH, low-molecular-weight heparin; PPC, prothrombin complex concentrate. | |
• Aspirin does not need to be stopped for low- and moderate-risk procedures. Aspirin should be held for 5 days prior to a high-risk procedure.
• Clopidogrel should be held for 5 days prior to all procedures.
• Most direct oral anticoagulants (DOACs) should be stopped 2 to 3 days before a procedure.
• Unfractionated heparin can be stopped approximately 6 hours prior to a procedure.
• Enoxaparin (low-molecular-weight heparin) should be held for 24 hours prior to all procedures.
• Warfarin should be held for 3 to 5 days before a procedure. INR should be monitored and brought below 2 (for low-risk procedures) or below 1.5 (for moderate- and high-risk procedures). For patients at high risk for thrombotic complications, this may require bridging with a shorter-acting agent such as enoxaparin or heparin.
When stopping anticoagulation, discuss with the referring service the risks of holding the medication versus the risk of bleeding associated with the procedure. In some cases, holding a medication can be dangerous. For example, holding an antiplatelet agent in the setting of a recently placed coronary stent can lead to stent thrombosis. In some cases, rules on holding medications or the acceptable thresholds for coagulation labs can be bent, but this is at the discretion of the attending who will be doing the case. When exceptions are made, it should be documented in the electronic medical record (EMR) and discussed with the patient and referring service.
Aspiration Risk
Aspiration risk is another important consideration prior to any interventional procedure, as most are performed using moderate sedation. Narcotic pain medication given during the procedure slows gastric motility and can be associated with nausea and vomiting. This introduces a risk of aspiration, especially with the patient lying flat on the table.
The best way to reduce the risk of aspiration is to keep the patient’s stomach empty. The American Society of Anesthesiologists recommends a minimum of 2 hours fasting for clear liquids and 8 hours of fasting for meals, though institutional guidelines for moderate sedation may vary.
Contraindications to Contrast
Severe allergic reactions to low osmolar, nonionic contrast agents are uncommon, occurring in less than 1 in 2,000 administrations. Risk factors for contrast reaction include prior reaction, unrelated allergies, asthma, anxiety, and usage of β-blockers. Of these, only a prior reaction to the same class of contrast medium is significant enough to prompt premedication or simply avoidance of intravenous contrast. Shellfish allergy or allergy to other iodine-containing compounds should not be considered a contraindication to intravenous contrast. Reactions to iodinated contrast are not considered true allergies, since they are thought to be mediated by direct histamine release rather than circulating antibodies. The mechanism of histamine release provides the theoretical basis for contrast premedication. For elective cases, a 13-hour oral premedication is recommended, consisting of 50-mg prednisone at 13, 7, and 1 hour before the procedure, plus 50 mg of diphenhydramine within an hour of the procedure. For urgent cases, intravenous premedication can be performed with 40 mg of methylprednisolone (or equivalent) given every 4 hours until contrast administration, and 50 mg diphenhydramine within one hour of the procedure. Even in urgent cases, premedication should be attempted for a minimum of 4 to 5 hours if at all possible. In emergent situations, involvement of anesthesia in anticipation of an anaphylactic event may be required if there are no alternatives to the use of iodinated contrast.
Iodinated contrast can also adversely affect renal function. Risk factors for contrast-induced nephropathy (CIN) include pre-existing chronic kidney disease, diabetes, heart failure, older age, and large contrast volume administered. Patients at risk should be screened with a serum creatinine level prior to contrast administration. Those with acute kidney injury, or an estimated glomerular filtration rate (GFR) of less than 60 should have the procedure delayed or performed with alternatives to iodinated contrast if possible. Hydration with intravenous fluids prior to and following the procedure can also decrease the risk, with specific protocols varying according to institutional guidelines.
Unstable Patients and the Risk of Decompensation
If the patient is unstable or if there is a significant chance the patient might decompensate in the IR suite, anesthesiology should be involved. Other situations in which general anesthesia may be appropriate include those patients with a significant tolerance to opioids, when the procedure is expected to cause a great amount of pain, or if the patient has severe cardiopulmonary disease.
Should the Patient Be Seen Before Being Brought to IR?
One of the major advantages of being an image-guided procedural specialty is that deciding whether to do a case or not can often be done with the clinical information and imaging available to us in the EMR. While we absolutely encourage it, going to see every consult prior to the patient being brought to the preprocedural area is not always necessary. On the other hand, doing so allows you to ask the patient questions, confirm information in the chart, do a focused examination, and make sure the patient is an active participant in the plan prior to obtaining consent.
Once you’ve progressed through the consult checklist and all of the necessary information is gathered, you will present the case to an attending. He or she will determine if the case is approved. If approved, let the referring service know and inform the staff in charge of scheduling. If the case is turned down, politely notify the referring team of the reason for the decision, if there is a plan for IR to follow the patient, and then document it in the patient’s chart. Generally speaking, consult notes and H&Ps in IR should be focused and to the point. The note communicates the information that was gathered and the plan that was formulated.
In the eyes of referring services, IR has gained a reputation of being able to think outside of the box and find creative ways to solve problems. For this reason, it is not uncommon to encounter some resistance when turning down a procedure. Always be courteous, stick to the facts, and involve your attending in the conversation early to ensure all parties are satisfied with the plan. Remember that the referring physicians just want the best care for their patient and may see IR as the patient’s last hope.
1.2 Preprocedure Tasks
Consent
After a case is approved and scheduled, the patient is usually brought to a holding area where nurses check the patient in, take vitals, draw blood if necessary, etc. This is also the time to consent the patient for the procedure if not done already. Depending on the policy at your institution, consents may be done by a resident, a nurse practitioner or physician assistant, a fellow, or the attending. Components of a good consent include a description of the procedure, the risks involved, the benefits, and the alternatives. Consent should also be obtained for any related procedures that might be necessary. For example, when consenting a patient for a lung biopsy, also consent for a chest tube in case the procedure is complicated by a pneumothorax.
Antibiotics
Some IR procedures require the patient to receive a dose of antibiotics before getting started. For the most part, vascular procedures are considered clean and do not require antibiotic prophylaxis. The rationale behind prophylaxis in certain circumstances has to do with contamination by the passage of needles and catheters through contaminated parts of the body and into the bloodstream.
Procedures involving the hepatobiliary, genitourinary, or gastrointestinal systems are considered contaminated, and require prophylactic antibiotics with gram-negative coverage. In some cases, antibiotics may be appropriate even in the absence of contamination, such as with placement of a stent-graft or for an embolization procedure. A stent-graft introduces an intravascular foreign body, and an embolization creates an infarcted area of tissue, both of which could be a nidus for bacterial growth. Procedures involving the spine rarely lead to infection, but it can be quite serious when it happens, often requiring surgical debridement. For this reason, vertebroplasty/kyphoplasty and discography patients receive prophylaxis to cover skin flora. Prophylaxis is not routinely recommended for permanent vascular access, including tunneled lines and chest ports, but some IR departments include it as part of their policy, since many of these patients are on immunosuppressive agents or are otherwise above average risk for infection.
As there is a lack of strong, prospectively validated data on the subject, the use of prophylactic antibiotics is based on the SIR standards of practice consensus guidelines. In some cases, the data are not strong enough to offer a consensus, and the practice is instead based on departmental policies. ▸Table 1.4 breaks down the general guidelines for antibiotic prophylaxis, but note that the antibiotics used and the procedures for which they are indicated may differ from those at your institution.
Table 1.4 Antibiotic prophylaxis for common interventional procedures
Antibiotic | Procedures |
No antibiotic prophylaxis necessary | Diagnostic angiography, angioplasty, stenting, IVC filter placement |
Cefazolin | Vertebroplasty, lumbar discography, endograft placement, gastrostomy tubes, possibly permanent vascular access lines, and dialysis access interventions |
Ceftriaxone or other cephalosporin with gram-negative coverage | Biliary drainage, abscess drainage, nephrostomy tubes, chemoembolization |
Sedation
Before nursing staff brings the patient into the IR suite, the plan for sedation should be established. Conscious sedation is typically accomplished using midazolam and fentanyl for the majority of cases. A typical starting dose of midazolam is 0.5 to 1 mg, while the starting dose of fentanyl is 50 mcg. Additional doses of each can be given as necessary during the procedure but should be used conservatively. In general, midazolam is a safe medication, with very high doses required before problems arise. In the rare case when a patient’s respiratory or mental status deteriorates from oversedation, reversal agents are available for use. Flumazenil is given for midazolam overdose (benzodiazepine antagonist), and naloxone for opiate overdose.
Oral conscious sedation is safe for most patients. Occasionally, a patient will describe a reaction to sedation medications and there will be a question of whether the procedure can be done without it. For simple procedures, such as line placement, local anesthetic may be sufficient. Trying to perform more complicated procedures without sedation can be risky, as it is important to have the patient remain still while you are working. If there are situations in which sedation needs to be withheld, make sure an attending knows about this ahead of time. Cases that require general anesthesia will also need to be planned ahead of time.
1.3 The IR Suite
After all tasks are completed in holding and consent is obtained, the patient can be brought back to the IR suite. This is a strange environment for newcomers, and the tools used come with a learning curve. Developing an understanding of frequently used terminology, how to use the equipment, and the safety issues involved will facilitate more active participation in IR procedures.
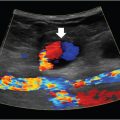
Fluoroscopy is the most commonly used imaging tool in IR. Ultrasound is predominantly used for vascular access and also for some percutaneous procedures. CT is used mainly for biopsies, drains, and ablations.
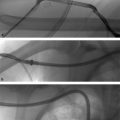
During fluoroscopically guided procedures, the patient lies on a table underneath an imaging detector, often called a “C-arm” (▸Fig. 1.1). The X-ray beam originates from the X-ray tube, which is located below the patient. The beam exits the tube, enters the patient, and passes through to the detector above the patient.
IR procedures are performed with pulsed fluoroscopy—essentially real-time X-rays. Pulsed fluoroscopy takes multiple individual X-rays per second, which appear to be “real-time” motion. Most procedures can be accomplished with surprisingly low frame rates of 4 to 7 frames per second; the lowest frame rate that allows the procedure to be accomplished in the quickest and safest manner should be selected. Contrast materials can be injected into vessels, ducts, organs, and spaces so that they can be visualized by fluoroscopy. The contrast will opacify the lumen or space by absorbing some of the X-rays on their way to the detector, allowing these structures to be visualized.