Most common malignant lesions
Primary lung cancer (adenocarcinoma, bronchoalveolar carcinoma, squamous cell carcinoma, small-cell lung cancer)
Primary pulmonary lymphoma
Primary pulmonary carcinoid
Pulmonary metastasis
Benign
Hamartoma, fibroma, chondroma, leiomyoma, lipoma
Infectious or inflammatory
Granuloma
Opportunistic infection
Rounded pneumonia
Abscess
Focal organizing pneumonia
Scar fibrosis
Necrobiotic nodule in rheumatoid arthritis
Wegener granulomatosis
Vascular
Pulmonary artery embolism
Pulmonary varices
Pulmonary arteriovenous malformation
Pulmonary infarct
Hematoma
Other
Intrapulmonary lymph node
Rounded atelectasis
Bronchogenic cyst
Mucoid impaction
Lung cancer is the third most common malignancy in both men and women in Germany, with 32,500 newly diagnosed cases in men and 14,600 in women each year. Lung cancer constitutes 14 % of malignant tumors in men and 7 % in women but accounts for a much higher proportion of all cancer deaths, 26 and 12 %, respectively. The proportionately higher death rates are attributable to the fact that lung cancer patients tend to have advanced disease at presentation and generally have a poor prognosis (overall 5-year survival rate of 15 %). The mean age at diagnosis is 69 years in both men and women (Robert-Koch-Institut und die Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. 2010).
A large autopsy study conducted in the USA identified pulmonary metastases in 20–54 % of all patients dying with a malignant neoplasm (Crow et al. 1981). This is much higher than the incidence of metastatic lung involvement at the time patients are first diagnosed with cancer. For obvious reasons, statistical data on the incidence of pulmonary metastases in the healthy population are hard to come by. The incidence reflects the prevalence of underlying primary tumors spreading to the lungs. Therefore, most pulmonary metastases are from colorectal cancer. A diagnosis of carcinoma of unknown primary (CUP) without identification of the primary tumor is very rare in the setting of whole-body MRI screening.
6.1.2 Pulmonary Nodules
A pulmonary nodule is defined as a more or less round lesion with a maximum diameter of 3 cm that is completely surrounded by normal lung (Figs. 6.1, 6.2, and 6.3) (Ost et al. 2003). Focal pulmonary lesions larger than 3 cm in size are generally considered indeterminate lung masses (Tan et al. 2003) and are much more likely to be malignant than smaller nodules (Winer-Muram 2006). Data obtained in larger CT screening populations suggest that, with a size threshold of 1–2 mm for the detection of pulmonary nodules, less than 5 % of all detected nodules with a diameter of up to 1 cm are malignant (Kim et al. 2002; Biederer et al. 2008). Based on the literature, it appears realistic to assume a threshold size of 3–4 mm for lung nodule detection with MRI performed at 1.5 T (The National Lung Screening Trial Research Team 2011). The sensitivities reported for MRI are greater than 80 % for pulmonary nodules measuring 5–10 mm and nearly 100 % for nodules larger than 10 mm (Girvin and Ko 2008; Feuerstein et al. 1992; Kersjes et al. 1997).
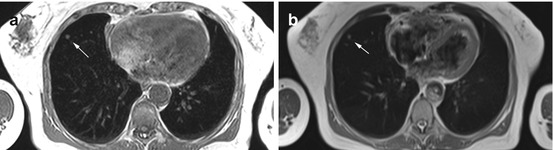
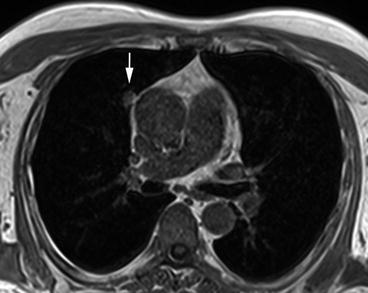
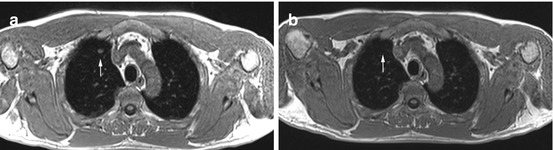

Fig. 6.1
Solitary pulmonary nodule (6 mm) in the right middle lobe in a 69-year-old female subject on T1w image (a) and on T2w HASTE image (b)

Fig. 6.2
Solitary pulmonary nodule (12 mm) in a 72-year-old male subject. The nodule is located near the pericardium and contiguous with the pleura

Fig. 6.3
Incidental solitary pulmonary nodule with follow-up MRI in a 49-year-old male subject. T1w screening image shows a 9-mm nodule in the right upper lobe (a). No lesion is detectable on follow-up MRI performed 3 months later (b)
6.1.2.1 The Solitary Pulmonary Nodule
Most solitary pulmonary nodules are benign. Eighty percent of all benign nodules are granulomatous inflammatory processes and lymph nodes (Kradin et al. 1985), 10 % are hamartomas, and 10 % other rare entities (Beigelman-Aubry et al. 2007). Nevertheless, some are early lung cancers or metastases (Fig. 6.4). In subjects with no history of malignancy, the only generally accepted criteria for ruling out a malignant nodule are age under 30 at the time of diagnosis and the presence of intranodular fat (hamartoma, lipoid pneumonia) (Diederich and Das 2006). With CT, certain calcification patterns suggest benign pulmonary nodules. With MRI, however, calcification is difficult to detect, and the interpreting radiologist therefore cannot make use of benign calcification patterns to rule out malignancy. The ability of MRI to demonstrate intranodular fat varies with the pulse sequence used and the size of the nodule. Since typically only non-contrast-enhanced chest images are obtained, contrast enhancement is another criterion not available for differentiating between benign and malignant nodules in the screening situation. Hence, demographic factors (age, pack years) are crucial in interpreting screening findings, while morphologic imaging features (well-defined versus ill-defined, smooth versus irregular contour) and localization within the lung (apical versus basal or peripheral versus central) can be considered but will not reliably rule out malignancy in many cases. This is because most pulmonary nodules seen on screening MRI are small, and the reliability of morphologic features decreases with lesion size.
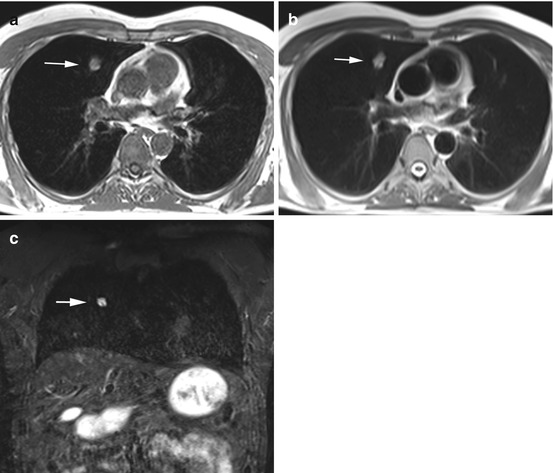

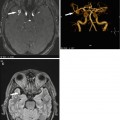
Fig. 6.4
Primary lung cancer in a 56-year-old male subject. Images demonstrate a solitary pulmonary nodule (20 × 14 mm) in the right middle lobe (a–c). Invasive diagnostic workup with CT-guided core biopsy yielded the diagnosis of primary lung cancer without nodal or parenchymal metastasis
Clinical Management
CT continues to be the method of choice for the workup of indeterminate pulmonary nodules. Solitary pulmonary nodules larger than 10 mm in diameter require further diagnostic evaluation by contrast-enhanced CT, PET, and/or biopsy. Lesions up to 10 mm in size should be followed up by unenhanced CT scans, ideally using computer-assisted volumetric analysis. Solitary pulmonary nodules smaller than 5 mm can be left alone, except in the setting of screening programs for high-risk groups. Less than 1 % of these very small nodules are malignant (Table 6.2) (Diederich and Das 2006; Henschke and Yankelevitz 2008; MacMahon et al. 2005).
Table 6.2
Recommended management strategy for the solitary pulmonary nodule detected by MRI screening in a healthy study population (Diederich and Das 2006)
Nodule size (mm) | Recommended follow-up |
|---|---|
<5 | No follow-up needed (<1 % malignant) |
5–10 | Interval screening using non-contrast-enhanced CT |
>10 | Contrast-enhanced CT, PET, and/or image-guided biopsy |
6.1.2.2 Multiple Pulmonary Nodules
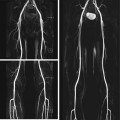
If multiple pulmonary nodules are detected, the rule of thumb is that each lesion must be evaluated individually (Fig. 6.5). The presence of multiple nodules makes a granulomatous or metastatic etiology more likely (Laurent and Remy 2002).
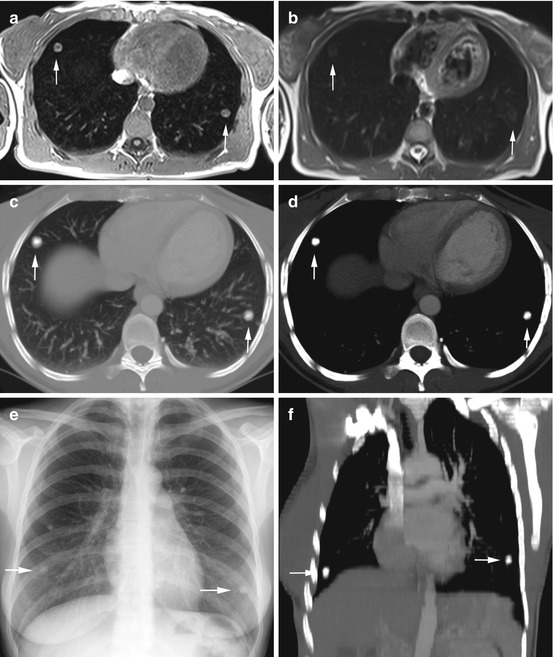

Fig. 6.5
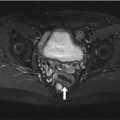
Incidental multiple pulmonary nodules with diagnostic workup in a 37-year-old female subject. T1w image shows a 13-mm lesion in the right middle lobe and a 12-mm lesion in the left lower lobe (a); the lesions have high-signal-intensity rims with less intense centers. The lesions are difficult to see on T2w HASTE image (b). Workup with CT (c, d, f) and conventional radiography (e) demonstrates homogeneously calcified nodules. Diagnostic resection (VATS) confirmed posttuberculous granulomas
6.1.3 Focal Pulmonary Masses >3 cm
Even larger pulmonary masses may not exhibit morphologic features that allow them to be reliably classified as benign or malignant; however, there are several features that are helpful in arriving at a characterization. Unfortunately, some criteria that can be assessed on CT scans, attenuation values, for example, are not available to the radiologist interpreting MR images. Solid and nonsolid lesions cannot be distinguished with certainty. Patterns of calcification that indicate benign pulmonary lesions on CT scans are only rarely seen on MR images (Fig. 6.6). The fluid sensitivity of T2-weighted pulse sequences may, however, allow differentiation between cystic lesions and solid nodules. Some lesions have a characteristic imaging appearance. These include arteriovenous malformations, aspergillomas in preexisting cavities, rounded atelectasis, bronchoceles, and circumscribed mucus retention (Beigelman-Aubry et al. 2007). While a smooth, well-defined contour is suggestive of benignity (Ost and Fein 2000), 21 % of malignant pulmonary nodules, especially metastases, have well-defined margins (Erasmus et al. 2000). An ill-defined, irregular, or spiculated contour is typically associated with malignant growth, but it may also be seen in focal organizing pneumonia (FOP), some forms of vasculitis (e.g., Wegener and lymphomatoid granulomatosis), and other granulomatous diseases (tuberculosis) (Kohno et al. 1993; Zwirewich et al. 1991). Cavitation is more frequently seen in malignant lesions but is also typical of pulmonary abscess and other granulomatous inflammatory processes (Woodring and Fried 1983). Intralesional fat is diagnostic of a benign nodule. Most fat-containing masses are hamartomas; less common are fatty granuloma, lipoma, and lipomatoid pneumonia. Hamartomas, however, tend to be smaller than 25 mm (Siegelman et al. 1986).
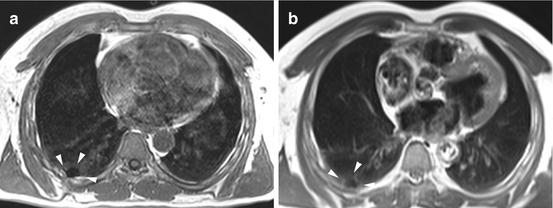

Fig. 6.6
Pleural calcinosis in a 68-year-old male subject. Post-inflammatory focal calcification of the posterior pleura can be identified by absence of signal on T1w image (a) and T2w image (b)
Clinical Management
Masses larger than 3 cm in diameter are much more likely to be malignant compared with smaller pulmonary nodules. Each of these masses requires diagnostic workup using contrast-enhanced CT, PET, and/or biopsy. When lung cancer is suspected, the radiologist must also report all imaging information relevant for TNM staging.
6.2 Lymphadenopathy
6.2.1 Introduction
Older studies report an incidence of lymphadenopathy of less than 1 % per year in family practices (Allhiser et al. 1981; Fijten and Blijham 1988). Twenty-five percent of patients have generalized lymphadenopathy, and 50 % have isolated cervical lymphadenopathy (Allhiser et al. 1981; Abba et al. 2002). According to a study from the Netherlands, in 1.1 % of cases, lymph node enlargement is due to an underlying malignant disease (Fijten and Blijham 1988).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree