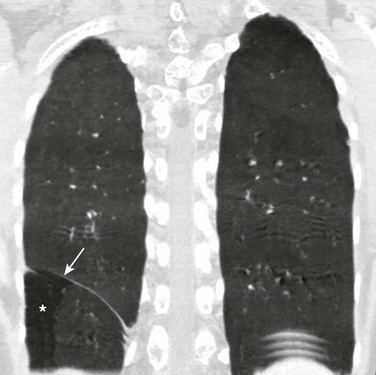
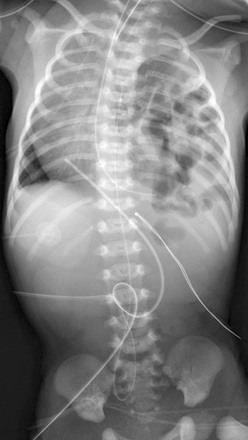
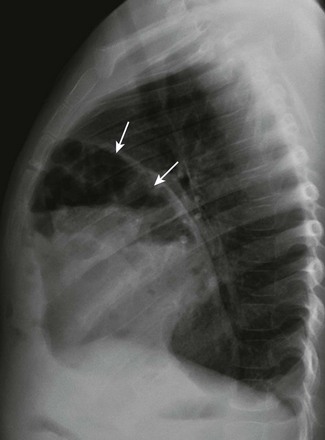
Chapter 61 The diaphragm is a dome-shaped musculofibrous membrane that separates the thoracic from the abdominal cavity. It also performs an important function in respiration. The diaphragm has a fibrous portion centrally (i.e., a central tendon) surrounded by a peripheral muscular portion. The diaphragm has three major musculofibrous groups, based on their origins: sternal, lumbar, and costal. Major structures pass through three openings: the caval opening (for the inferior vena cava and some branches of the right phrenic nerve), the esophageal hiatus (for the esophagus, anterior and posterior vagal trunks, and some small esophageal arteries), and the aortic hiatus (for the aorta, azygous vein, and thoracic duct). The diaphragm is innervated by the phrenic nerve, which is formed from the central nerves of C3, C4, and C5.1,2 Diaphragmatic lesions can arise from a variety of congenital, traumatic, infectious, and neoplastic conditions, as discussed in the following sections. Duplication of the Diaphragm (Accessory Diaphragm) Etiology: Duplication of the diaphragm, also known as an accessory diaphragm, is a rare congenital anomaly. It is almost always located on the right side and frequently is associated with lobar agenesis-aplasia complex.3,4 Although the precise pathogenesis of duplication of the diaphragm is currently unknown, it is suggested that this anomaly is the result of a defect of synchronization between the caudal migration of the septum transversum and the development of the bronchial system.3,5 Instead of developing independently, these two structures mutually interfere in each other’s growth. In gross pathology, the accessory diaphragm is a fine fibromuscular membrane with a serosal lining that is united to the anterior part of the diaphragm.3 It follows a posterosuperior direction to join the posterior chest wall, separating the right hemithorax into two parts.3,4 It is crescentic in form and usually has an opening (i.e., central hiatus) medially, through which vessels and bronchial structures pass. Affected patients may be asymptomatic, but most present with respiratory difficulties of varying degrees.5 Imaging: On chest radiographs or computed tomography (CT), the accessory diaphragm may have two different appearances.3 When the central hiatus is markedly narrowed and the trapped lung is not aerated, it appears like a mass. However, when the trapped lung is aerated, the accessory diaphragm is seen as a fissurelike structure in the right base extending from the anterior aspect of the hemidiaphragm cephalad toward the posterior chest wall. On CT, it is seen as a bandlike structure with crowding of pulmonary structures as the bronchi and vessels traverse the central hiatus (Fig. 61-1).4,5 Traditionally, congenital diaphragmatic hernias (CDHs) have been classified according to their anatomic location. Almost 90% of CDHs are reported to involve the posterolateral aspect of the diaphragm and are referred as to Bochdalek hernias.6 Nonposterolateral CDHs occur most often in the anterior portion of the diaphragm and are known as Morgagni hernias. However, diaphragmatic defects do not exclusively localize to these two areas, and thus some defects do not follow this classification. To further complicate the classification, some diaphragmatic hernias have a sac, which is thought to represent focal thinning of the diaphragmatic musculature. Thus the terms sac hernia and eventration currently are poorly defined. Despite its limitations, an anatomic-based classification system continues to be used (Box 61-1).6 Etiology: CDH of the Bochdalek type is a birth defect that is associated with significant morbidity and mortality.7 The average prevalence of CDH, derived from a meta-analysis of 16 population-based studies, is 1 in 4000 births.7 The Bochdalek hernia is the most common subtype, accounting for approximately 90% to 95% of all CDHs.6 Approximately 85% of these hernias occur on the left side, whereas right-sided and bilateral hernias represent only 13% and 2% of cases, respectively.8 Bochdalek hernias can be divided into two main categories—isolated and complex—based on the presence of additional associated malformations.6 The underlying pathogenesis of CDH is poorly understood. However, much more is currently known about the cellular events and molecular cues that control early differentiation of the diaphragm. The diaphragm initially develops as a septum between the heart and the liver, progresses posterolaterally, and closes at the Bochdalek foramen at approximately 8 to 10 weeks of gestation.8,9 Studies have suggested that the primary abnormality resulting in a Bochdalek hernia is failure or delay of the pleuroperitoneal fold and transverse septum to properly fuse with the intercostal muscles around the eighth week of gestation.9 More recent evidence suggests that a diaphragmatic hernia and lung hypoplasia are associated, but they may not be causally related.8 Imaging: A Bochdalek hernia may appear at birth as an opacified hemithorax with contralateral cardiomediastinal shift. As the infant swallows air, the air-filled gut located within the hemithorax may become apparent (Fig. 61-2). In the case of intraabdominal solid organ herniation such as the liver and spleen, the hemithorax can remain homogeneously opacified. When large and with herniation of the bowel, a paucity of air in the abdomen causing the so-called “scaphoid abdomen” can be seen on abdominal radiographs.9 The position of catheters and tubes is helpful in confirming the presence of a Bochdalek hernia. The nasogastric (NG) tube deviates to the side opposite to the hernia in the chest. If the stomach is herniated within the hemithorax, the tip of the NG tube can project in the chest. The position of umbilical venous catheters also is affected according to the location of the liver, which is shifted either in the abdomen or chest. In contrast, the position of umbilical arterial catheters is rarely affected because of their retroperitoneal location.10 In the postoperative period, an ipsilateral pneumothorax is a common finding and should not be rapidly evacuated. A rapid evacuation of a pneumothorax in this situation may cause mediastinal rotation and subsequent venae cavae obstruction because of the increased mobility of the neonatal mediastinum.9 The pleural air subsequently reabsorbs by itself and sometimes is replaced by fluid. Ultrasound with color Doppler may help delineate the venae cavae and the hepatic vasculature, and it may identify the presence of herniated solid organs before surgery. CT and magnetic resonance imaging (MRI) may play an occasional role in excluding a congenital lung anomaly such as congenital pulmonary airway malformation or pulmonary sequestration.9 Treatment and Follow-up: Treatment of all types of CDH, including the Bochdalek hernia, can be classified into medical or surgical management. The medical management of CDH focuses on addressing the underlying major causes of neonatal death as a result of CDH, such as pulmonary hypoplasia and pulmonary hypertension. It includes the use of extracorporeal membrane oxygenation, high-frequency ventilation, and inhaled nitric oxide.11–13 Surgical repair of CDH is performed with a transabdominal or transthoracic approach, and more recently, surgery is performed laparoscopically or thoracoscopically. The herniated abdominal viscera are removed from the chest and repositioned in the abdomen. The posterolateral diaphragmatic defect is usually closed with nonabsorbable sutures if the defect is small or with a prosthetic patch if the defect is larger than 5 cm.14 Currently no specific guideline exists for following up on children with a repaired CDH. However, chest radiographs often are obtained routinely for confirmation of an intact diaphragm and early detection of a possible CDH recurrence. On follow-up chest radiographs, abnormalities including persistent lung hypoplasia, decreased pulmonary vascularity, and mediastinal shift may be observed. Etiology: The foramen of Morgagni is an anterior opening in the diaphragm that extends between the sternum medially and the eighth rib laterally. In Morgagni hernias, also known as retrosternal hernias, the underlying congenital defect results from developmental failure of the fibrotendinous elements of the sternal part of the diaphragm to fuse with the costal part.15–17 They account for 9% to 12% of the diaphragmatic defects in infancy. These hernias usually are unilateral and are right sided in 90% of cases.9 A Morgagni hernia may occur as one of the components of the pentalogy of Cantrell, which is characterized by omphalocele, anterior diaphragmatic hernia, sternal cleft, ectopia cordis, and intracardiac defect such as a ventricular septal defect or a diverticulum of the left ventricle.9,17 A Morgagni hernia can be seen in association with congenital heart disease, intestinal malrotation, and chromosomal abnormalities, most frequently Down syndrome.9,17 Imaging: Most cases of Morgagni hernias are discovered incidentally on chest radiographs that are obtained for evaluation of other conditions in older children and adults. On chest radiographs, the diagnosis is made when anterior herniation of bowel loops is identified on the lateral chest radiograph (Fig. 61-3). When solid organs such as the liver or spleen are involved, the appearance may not be specific and can resemble focal diaphragmatic eventration, lymphadenopathy, or a foregut duplication cyst. Ultrasound, CT, or MRI can be helpful in the diagnosis when solid organs are herniated.9,16,17 Treatment and Follow-up: The current treatment of choice for a Morgagni hernia is surgical repair at the initial diagnosis, even in the absence of symptoms, because of the increased risk of developing bowel obstruction and subsequent incarceration. Most Morgagni hernias can be repaired laparoscopically.1,9,16 Etiology: Approximately 5% to 20% of pediatric patients may present with a delayed CDH.18 Although currently it is not clear whether the diaphragmatic defect in these patients is congenital or acquired, it has been assumed that the defect may have been present prenatally but was either small or temporarily occluded by solid organs such as the liver or spleen.18,19 In a recent multicenter retrospective study by the Congenital Diaphragmatic Hernia Study Group,19
The Diaphragm
Overview
Congenital Anomalies
Congenital Diaphragmatic Hernia
Congenital Diaphragmatic Hernia: Bochdalek
Congenital Diaphragmatic Hernia: Morgagni
Delayed Presentation of Congenital Diaphragmatic Hernia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree