Frequency
Incidence, <15/100,000 women/year
Age predilection
Mean age at presentation, 51 years
Risk factors
HPV infection, smoking
Type of lesion
Malignant
Signs and symptoms
Vaginal discharge, spotting, abnormal vaginal bleeding, painful defecation and/or bladder voiding
Differential diagnosis
Endometrial cancer, cervical sarcoma, uterine fibroid
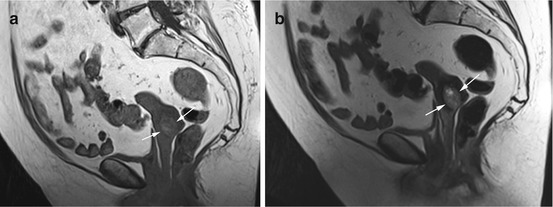
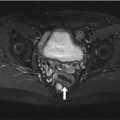
Fig. 12.1
(a) Sagittal T1w image shows distension of the cervix and an inhomogeneous mass of high signal intensity (arrows). (b) Sagittal T2w image confirms the high-signal-intensity cervical mass (arrows). The findings are suspicious for cervical cancer. Also seen is diverticulosis
Clinical Management
Thickening of the cervical wall or localized lesions should be evaluated further, ideally using transvaginal ultrasound and contrast-enhanced MRI (Scheidler and Heuck 2002).
12.1.2.2 Nabothian Cysts
Nabothian cysts are common, benign retention cysts that develop when one of the mucous glands of the cervix becomes obstructed. There is an association with chronic inflammatory conditions of the cervix and with multiparity (Table 12.2). Nabothian cysts range in size from a few millimeters to 4 cm. Women typically have multiple cysts located along the cervix. The cysts are very bright on T2-weighted images, while they are isointense or slightly hyperintense (protein content) on T1-weighted images. The most important differential diagnosis is malignant adenoma, a subtype of cervical cancer with an imaging appearance characterized by multiple cysts with a soft-tissue component. Unlike a nabothian cyst, malignant cervical adenoma enhances after administration of contrast medium (Okamoto et al. 2003; Togashi et al. 1987).
Table 12.2
Nabothian cysts
Frequency | Very common |
Age predilection | Before menopause |
Location | Uterine cervix |
Type of lesion | Benign |
Signs and symptoms | Asymptomatic |
Differential diagnosis | Cervical cancer (malignant adenoma) |
Clinical Management
Most nabothian cysts are asymptomatic and benign, and there is no need to report them (Okamoto et al. 2003).
12.1.3 Uterus
The most common uterine findings are anomalies and tumors of different etiologies. They are discussed in this section along with endometriosis.
12.1.3.1 Anomalies
The normal uterus is a pear-shaped organ located between the urinary bladder and the rectum. The nonpregnant uterus is 7 cm long. A severe congenital anomaly such as absence of the uterus is usually known to the woman. In older women, an absent uterus is more likely due to hysterectomy. Relevant uterine anomalies that may be detected by screening MRI result from defective fusion or resorption and include bicornuate uterus (double uterus with or without a double cervix), duplex uterus (bicornuate uterus with two vaginas), and (sub-)septate uterus (persistent median septum, which completely or partially divides the cavity). The prevalence is approx. 1 % (Homer et al. 2000). MRI is highly sensitive and specific in demonstrating uterine anomalies, with a fat-suppressed T2-weighted pulse sequence being especially useful.
Clinical Management
Uterine anomalies may impair fertility and should therefore be communicated to younger women who wish to have children (Troiano and McCarthy 2004).
12.1.3.2 Tumors
This section discusses uterine fibroids or leiomyomas and endometrial cancer, which are, respectively, the most common benign and malignant tumors of the uterus.
12.1.3.2.1 Uterine Fibroids
Uterine fibroids are the most common gynecologic tumors in women of reproductive age (Table 12.3). In autopsy series, fibroids were found in up to 77 % of women with the incidence increasing with age, most markedly after age 40. This is because their development and growth is hormone dependent (Cramer and Patel 1990; Flake et al. 2003). Uterine fibroids are round to oval lesions consisting of smooth muscle cells, collagen, extracellular matrix, fat, and fibrin. Women usually have multiple fibroids. Fibroids are found within the endometrial cavity, in the uterine wall, or extending beyond the uterus. Diffuse presence of fibroids within the myometrium is referred to as myomatous uterus. MRI is considered the most suitable imaging modality for detecting and characterizing uterine fibroids. On T2-weighted images, fibroids are usually conspicuous as smooth and sharply demarcated homogeneous masses of low signal intensity compared with the surrounding myometrium (Fig. 12.2). On T1-weighted pulse sequences, they have the same signal intensity as the myometrium. Intralesional calcifications appear dark and are quite common. Degeneration of fibroids is also common and is associated with the presence of intralesional hemorrhage (high T1 signal intensity, variable T2 signal intensity), cystic portions (high T2 signal intensity), or mucinous degeneration (very high T2 signal intensity) (Fig. 12.3). Fibroids can exhibit homogeneous contrast enhancement (Murase et al. 1999). Uterine fibroids must be distinguished from adenomyosis (a form of endometriosis formerly known as internal endometriosis). Adenomyosis is the presence of ectopic endometrial glands in the myometrium. It is usually diffuse but may also be focal. Diffuse adenomyosis is suggested by widening of the junctional zone (>12 mm) and is best evaluated on T2-weighted images. The junctional zone has low T2 signal intensity, delineating it from the endometrium (high signal intensity) and the myometrium (intermediate signal intensity). Areas of adenomyosis are characterized by low T2 signal intensity interspersed with bright foci. Focal forms of adenomyosis appear as poorly marginated areas of low signal intensity that are less well defined than fibroids (Murase et al. 1999). Other differential diagnoses are focal myometrial contraction (which can be ruled out by serial imaging) and leiomyosarcoma.
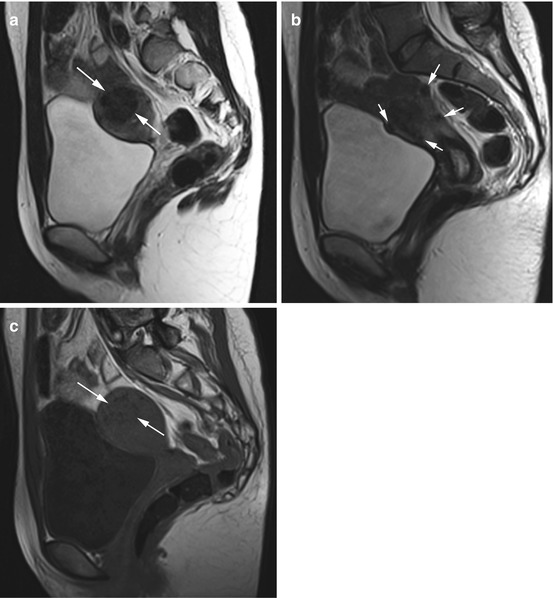
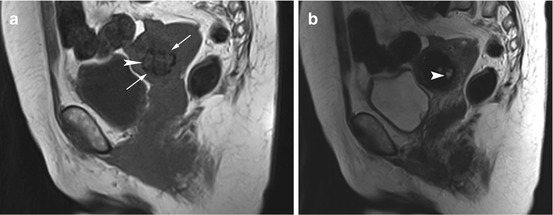
Table 12.3
Uterine fibroids
Frequency | Incidence, approx. 80 % |
Age predilection | >25 years, no growth after menopause |
Type of lesion | Benign, <0.2 % risk of malignant transformation |
Signs and symptoms | Present in only 25–50 % of cases; abnormal periods, pain, impaired fertility |
Differential diagnosis | Adenomyosis, myometrial contraction, leiomyosarcoma |

Fig. 12.2
(a) Sagittal T2w image reveals a well-defined, homogeneous mass of low signal intensity in the uterine myometrium (arrows) in a 27-year-old subject. (b) Sagittal T2w image demonstrates additional rounded masses, which are subserosal in location (arrows). (c) Sagittal T2w image again shows the large intramural mass, which is slightly hypointense (arrows). In summary, MRI shows a myomatous uterus with a large intramural fibroid and multiple subserosal fibroids

Fig. 12.3
(a) Sagittal T1w image reveals a rounded low-signal-intensity mass with slightly irregular borders in the uterine wall (arrows) in a 54-year-old woman. In addition, the image shows patchy hyperintensities (arrowhead). (b) Sagittal T2w image shows the hyperintense areas more clearly (arrowhead). In summary, the appearance is consistent with an intramural fibroid with hemorrhagic degeneration
Clinical Management
Uterine fibroids can cause abdominal pain and heavy menstrual bleeding. Some forms compromise fertility and/or increase the risk of miscarriage, which is why younger women should be informed when uterine fibroids are detected by MRI screening (Murase et al. 1999). This is different in postmenopausal women, who need only be informed when very large fibroids are found or when there is diffuse involvement with a clear mass effect on adjacent organs (urinary bladder, rectum).
12.1.3.2.2 Endometrial Cancer
Endometrial cancer is diagnosed in 11,700 women in Germany each year. The annual incidence is 20–30 new diagnoses per 100,000 women and is on the rise. It is the fourth most common cancer in women, accounting for 5.7 % of all cancers in women, and the most common cancer of the female reproductive organs. Most uterine cancers arise in the endometrium with over 90 % being adenocarcinomas. The mean age at presentation is 68 years (Table 12.4) (Robert-Koch-Institut und die Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. 2008). MRI has limitations in detecting early endometrial cancer (especially when imaging protocols without contrast medium administration are used). Depending on the stage of endometrial cancer, the appearance on MR images may range from minimal changes to massive enlargement of the uterus. On T1-weighted images, endometrial cancer is isointense or hypointense relative to the endometrium or myometrium. On T2-weighted images, the cancer is isointense or hypointense to endometrium and usually slightly hyperintense relative to myometrium. The higher cellularity of endometrial carcinomas results in high signal intensity on diffusion-weighted images with lower ADC values. Following administration of contrast medium, endometrial cancer enhances earlier than normal endometrium and is hypointense to the myometrium (Namimoto et al. 2009; Sala et al. 2007). Several entities have to be considered in the differential diagnosis: endometrial hyperplasia or polyps (difficult to differentiate; DWI is helpful), fibroids, adenomyosis, and uterine sarcoma (2–6 % of malignant uterine tumors, typically larger and more heterogeneous).
Table 12.4
Endometrial cancer
Frequency | Incidence, 20–30/100,000 women |
Age predilection | Mean age at presentation, 68 years |
Risk factors | Endogenous and exogenous estrogen, obesity |
Type of lesion | Malignant |
Signs and symptoms | Irregular bleeding, pain |
Differential diagnosis | Endometrial hyperplasia, polyps, fibroids, endometrial sarcoma |
Clinical Management
A suspected malignant tumor of the endometrium/uterus must be disclosed, and the woman should undergo further diagnostic workup with endosonography or contrast-enhanced MRI (Nalaboff et al. 2001).
12.1.4 Endometriosis
Endometriosis is the ectopic occurrence of functional endometrial tissue including glands outside the uterine cavity. The severity is variable, ranging from microscopic ectopic cysts to so-called endometriomas, which are circumscribed endometriotic or chocolate cysts several centimeters in size. Endometriosis is typically seen during the reproductive years with a mean age at presentation of 25–29 years (Dmowski et al. 1997). The prevalence is 5–10 %, and up to 25 % of women suffering from endometriosis have lower abdominal pain (Table 12.5) (Eskenazi and Warner 1997; Olive and Schwartz 1993). It is assumed that endometrial tissue enters the peritoneal cavity through the fallopian tubes, a process known as retrograde menstruation. This assumption is supported by the anatomic distribution of endometriotic lesions, which are most commonly found on the ovaries (see next section) and along the uterine appendages and the uterus. Theoretically, ectopic endometriotic tissue may be found anywhere within the abdominal cavity. For correct interpretation of MR images, the examiner must be aware that ectopic endometrial tissue is subject to the same cyclic variation as within the uterine cavity. Ectopic endometrial tissue has a cystic appearance and is characterized by very high signal intensity on T1-weighted images (due to high protein and blood content). The appearance on T2-weighted images ranges from hyperintense to hypointense, depending on the amount and age of blood present (high iron content lowers T2 signal intensity). Following administration of contrast medium, the cyst walls show nonspecific enhancement (Sugimura et al. 1993; Siegelman and Outwater 1999). Malignant transformation of endometriotic tissue may occur but is very rare (<1 %). Endometriotic lesions must be differentiated from cystic tumors such as dermoids (which, unlike endometrial tissue, will show a drop in signal intensity on fat-suppressed images) and cystic ovarian cancer.
Table 12.5
Endometriosis
Frequency | Prevalence, 5–10 % |
Age predilection | Mean age at presentation, 28 years |
Risk factors | Environmental toxins may promote endometriosis |
Type of lesion | Benign, <1 % risk of malignant transformation |
Signs and symptoms | Cyclic cramping pain, dyspareunia, impaired fertility |
Differential diagnosis | Dermoid, ovarian cancer |
Clinical Management
Younger women, who are assumed to wish to have children, should be informed when endometriosis is detected, as 30–50 % of women with endometriosis are infertile (Eskenazi and Warner 1997).
12.1.5 Ovaries
Anomalies such as agenesis or multiple-anomaly syndromes, which either have no relevance or are so severe that affected women are aware of their condition, are not discussed here. Instead, we will focus on the more common ovarian lesions that may be detected by MRI including unilocular and multilocular ovarian cysts as well as solid or complex masses with variable cystic components.
Several morphologic MR features have been shown to be useful for characterizing ovarian lesions (Gore et al. 2010; Imaoka et al. 2006). The features include the site of the lesion and single versus multiple occurrences as well as the ratio of cystic to solid components (purely cystic, cystic with solid components, and predominantly solid).
Contrast enhancement is another important criterion for differentiating between benign and malignant ovarian lesions but is not available in screening MRI, which is typically performed without contrast medium. A number of features suggesting malignancy can be evaluated on unenhanced T1-weighted and T2-weighted images including a solid or predominantly solid appearance and, in the case of a cystic lesion, thickened cyst walls and multiple septa. Other criteria that may contribute to the differentiation include growth beyond the ovary, presence of ascites, and locoregional lymphadenopathy. When these criteria are applied in conjunction with contrast medium administration, MRI has over 90 % sensitivity and specificity in characterizing an ovarian mass (Hricak et al. 2000).
12.1.5.1 Unilocular Ovarian Cysts
The most common incidental findings are functional or retention cysts (Gore et al. 2010) (Fig. 12.4). They are frequently seen in premenopausal women but also occur during menopause (prevalence of 3.3–14.8 %) (Table 12.6). Functional ovarian cysts are fluid-filled cavities resulting from failure of the dominant follicle to ovulate (follicular cyst) or failure to regress (corpus luteum cyst).
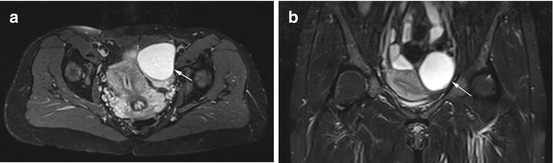

Fig. 12.4
Axial PD image (a) and coronal TIRM image (b) obtained in a 38-year-old woman show a smoothly marginated, homogeneous cyst of high signal intensity (arrow). The cyst has a size of up to 6.6 cm, and the appearance is consistent with a typical functional cyst
Table 12.6
Unilocular ovarian cysts
Frequency | Prevalence, 3–15 % |
Age predilection | Premenopausal |
Type of lesion | Benign |
Signs and symptoms | Usually asymptomatic |
Differential diagnosis | Serous cystadenoma, cystic ovarian cancer |
These cysts are usually unilocular simple cysts with a smooth margin and range in size from 1 to 8 cm. Corpus luteum cysts are often larger and may contain blood. Paraovarian cysts are rare simple cystic tumors (Fig. 12.5) arising from the mesothelium or embryonic ducts (Müllerian and Wolffian ducts). These cysts must be differentiated from the normal ovaries. A hydrosalpinx developing on the basis of salpingitis or endometriosis can also mimic an ovarian cyst. Serous cystadenoma (cystoma) is not easily differentiated from a functional cyst based on morphologic imaging features when it is unilocular and no solid portions are seen. Serial examinations are necessary to make the distinction.
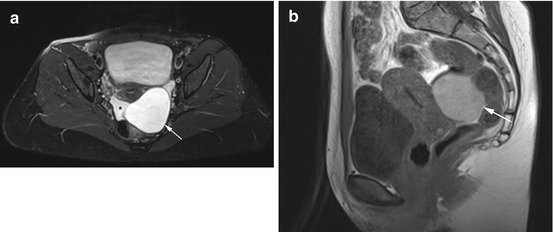

Fig. 12.5
Axial PD image (a) and sagittal T1w image (b) reveal a large, smoothly marginated cyst (arrow) in a 45-year-old woman partially surrounded by free fluid (asterisk). The cyst has high signal intensity, and there is a small, physiologic amount of free fluid in the rectouterine pouch. There is good demarcation of both ovaries. The appearance is consistent with a hemorrhagic paraovarian cyst
Clinical Management
Most ovarian cysts recede within 2 months in premenopausal women (up to age 45). Since there is a risk of rupture and since it is important to differentiate a cyst from serous cystadenoma, ovarian cysts larger than 2 cm should be reported and followed up. As no cyclic regulation occurs in postmenopausal women, the primary aim should be to initiate further diagnostic workup with transvaginal ultrasound and CA-125 tumor marker test (Oyelese et al. 2002; Dikensoy et al. 2007; McDonald and Modesitt 2006). Paraovarian cysts should be followed up by imaging.
12.1.5.2 Multilocular Ovarian Cysts
Polycystic ovaries can be encountered in healthy premenopausal women (Balen et al. 1995). Endocrine dysfunction presenting in the second or third decade of life may point to polycystic ovary syndrome (formerly known as Stein-Leventhal syndrome). This syndrome is characterized by the classic triad of obesity, hirsutism, and infertility (Fig. 12.6). In affected women, MRI demonstrates enlarged ovaries containing follicular cysts (<1 cm). The cysts typically occur in the periphery of the ovaries, giving the appearance of a string of pearls. Other multilocular cystic tumors are serous and mucinous cystadenomas (Fig. 12.7), which account for about 25 % of benign ovarian neoplasms. They are bilateral in 20 % of cases and tend to be more common after menopause (Dorum et al. 2005). Cystadenomas frequently contain septa and calcifications. Half of the women with endometriosis have ectopic endometriotic tissue on the ovaries. MR imaging demonstrates multiple endometriomas containing old blood (Fig. 12.8). The lesions are known as chocolate cysts and characteristically have high signal intensity on T1-weighted images and low signal intensity on T2-weighted images, a pattern known as T2 shading.
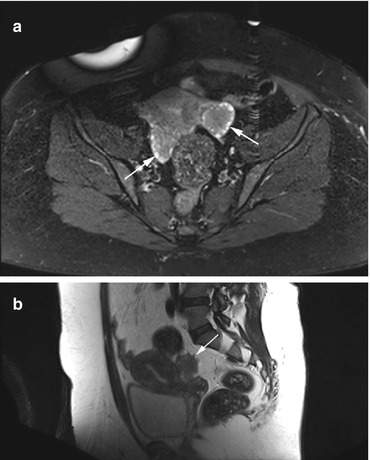
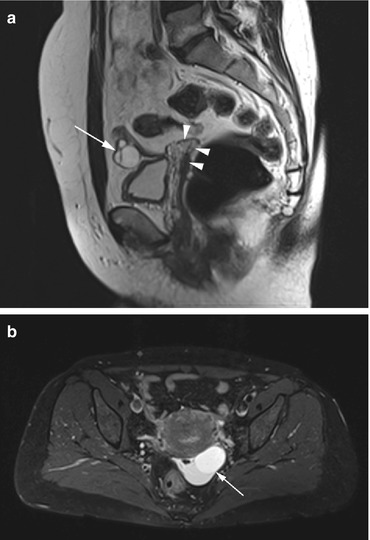
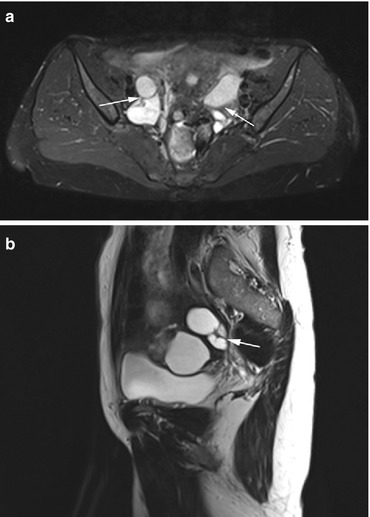

Fig. 12.6
Axial PD image (a) and sagittal T2w image (b) obtained in a 34-year-old obese woman. There are prominent ovaries with lower signal intensity in the center and follicular cysts arranged around the periphery of both ovaries, producing the string-of-pearls sign (arrows). The diagnosis is polycystic ovary syndrome

Fig. 12.7
(a) Sagittal T2w image of a 62-year-old woman with a history of hysterectomy (arrowheads) reveals a cystic ovary with multiple septa (arrow). The diagnosis is cystadenoma. (b) Axial PD image of a 45-year-old woman shows a septated cyst of mixed signal intensity (arrow)

Fig. 12.8
(a) Axial PD image of a 50-year-old woman shows multiple ovarian cysts (arrows) of variable signal intensity in both ovaries. (b) Sagittal T2w image demonstrates a hydrosalpinx with a tortuous course of the tube (arrow)
12.1.5.3 Mixed Cystic and Solid Ovarian Tumors
Primary ovarian cancer and metastases to the ovary (Figs. 12.9 and 12.10) are mainly solid but frequently have cystic spaces (Table 12.7). Ovarian cancer is most common in the fourth to seventh decade of life, and 90 % of ovarian cancers arise from the epithelium (Imaoka et al. 2006). The most common histologic types, in order of frequency, are serous and mucinous carcinoma, cystadenocarcinoma, endometrioid carcinoma, and undifferentiated carcinoma. Malignant germ cell tumors are very rare. Metastases usually involve both ovaries with gastric cancer being the most common primary tumor (e.g., Krukenberg tumor).
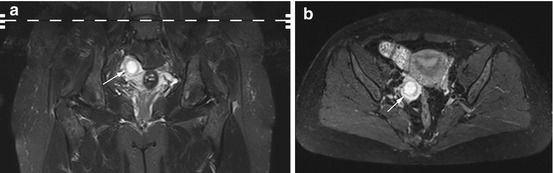
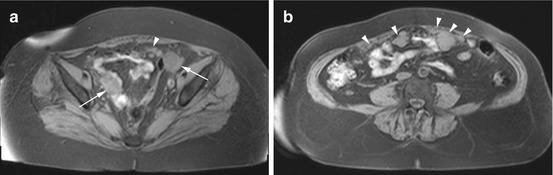

Fig. 12.9
Coronal TIRM image (a) and axial PD image (b) obtained in a 45-year-old woman with a partially cystic (center) and partially solid mass (periphery) in the right ovary (arrow). The diagnosis is cystadenocarcinoma

Fig. 12.10
T1w 3D FLASH images obtained in a 76-year-old woman demonstrate solid masses (arrows) in both ovaries (a) and multiple metastatic implants (arrowheads) in the peritoneal cavity (b). The findings are consistent with metastatic ovarian cancer
Table 12.7
Mixed cystic and solid ovarian tumors
Frequency | Incidence, 14/100,000 women/year |
Age predilection | 58–65 years |
Risk factors | Risk increases with the duration of the reproductive phase, genetic predisposition, childlessness |
Type of lesion | Malignant |
Signs and symptoms | Nonspecific, genital hemorrhage |
Differential diagnosis | Benign ovarian cyst, cystadenoma, dermoid |
Dermoid or mature teratoma is a benign tumor usually occurring before the second decade of life, which is why this tumor is unlikely to be encountered at screening (Gore et al. 2010).
12.2 The Male Genital Organs
12.2.1 Introduction
The penis and the scrotum constitute the external male genital organs, while the internal reproductive organs include the testicles with the epididymis and spermatic cords, the prostate, the seminal vesicles, and other accessory gonads.
Relevant abnormalities of the penis and scrotum are not expected to be seen in the setting of screening MRI as such changes would most likely have already been discovered by the affected man and led him to see a doctor.
12.2.2 Testicles
The most important testicular imaging findings are hydrocele, aberrations in number, abnormal size, cryptorchidism, and mass lesions.
12.2.2.1 Hydrocele
A hydrocele is a congenital or acquired collection of serous fluid in the space of the tunica vaginalis testis (Table 12.8). Detection of acquired hydroceles is relevant in the screening situation (Akin et al. 2004). They tend to occur in middle-aged or older men on one or both sides. A definitive pathomechanism has not been established (Woodward et al. 2003). A thin layer of fluid around the testis is normal and has been demonstrated in 86 % of men in an ultrasound study (Leung et al. 1984). Acquired hydroceles are usually idiopathic in origin and predominantly affect men after age 40. Less commonly, acquired hydroceles are associated with tumors, inflammation, or infection. Hydrocele is bilateral in 7–10 % of cases (Leung et al. 1984). It is depicted with very high contrast on fat-saturated T2-weighted images; usually there is no compression of the underlying testis (Fig. 12.11). In the differential diagnosis, hematocele and pyocele must be ruled out (Woodward et al. 2003).
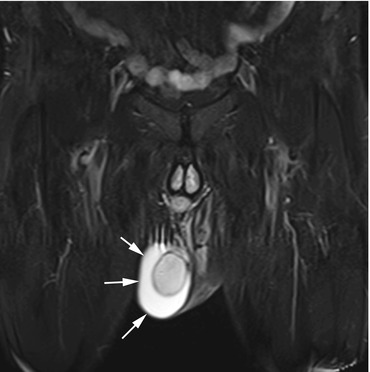
Table 12.8
Hydrocele
Frequency | Incidence, >15 % |
Age predilection | Acquired hydrocele, >40 years |
Type of lesion | Benign |
Signs and symptoms | Unusually asymptomatic; painless testicular swelling |
Differential diagnosis | Hematocele, pyocele |

Fig. 12.11
Coronal TIRM image demonstrates a crescent-shaped area of homogenous high signal intensity surrounding the right testis (arrows). This appearance indicates serous fluid in the tunica vaginalis testis and is typical of a hydrocele. The tunica albuginea is clearly delineated as a low-signal-intensity line around the testis
Clinical Management
There is no need to report an idiopathic hydrocele; treatment is necessary only when swelling and pain lead to problems, for which an affected man seeks treatment on his own (Gyapong et al. 2000).
12.2.2.2 Aberration in Number
Aberrations in number are easily identified and are usually known to a man. Polyorchidism is very rare, with about 100 cases reported in the literature worldwide. The most common form is the presence of three testicles within the scrotum. However, supernumerary testicles may also be located inguinally or intra-abdominally. It is still under debate whether surgical exploration or a conservative approach with serial imaging is the best management option in these cases (Sheah et al. 2004; Yeniyol et al. 2004; Chung and Yao 2002). On MR images, an accessory testicle has the same signal intensity as orthotopic intrascrotal testicles. A testicular tumor must be ruled out in the differential diagnosis.
The absence of one or both testicles from the scrotum is nearly always due to surgical removal, because of testicular cancer or testicular torsion, for instance. Nevertheless, a careful search for a cryptorchid testis should always be done in such cases.
Clinical Management
Polyorchidism should be reported and at least followed up by imaging (Bhogal et al. 2007). The absence of one or both testicles is usually known and requires no further workup.
12.2.2.3 Abnormal Size
A normal adult testis is 3–5 cm long, 2–4 cm wide, and about 3 cm in anteroposterior extension with marked interindividual variation in dimensions (Kim et al. 2007). An exact size threshold for diagnosing testicular atrophy does not exist. This is why both testicles are measured and atrophy is assumed when there is a significant difference (>50 %) in one or more dimensions between the two testicles (Fig. 12.12). When bilateral testicular atrophy is present, the size of both testicles is well below the normal range. Often, there is concomitant atrophy of the epididymis and vascular structures. Testicular atrophy may be due to cryptorchidism, postinflammatory changes, or trauma.
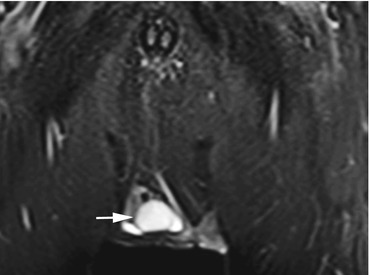

Fig. 12.12
Coronal TIRM image shows a slightly smaller testicle on the right (arrow) and a small hydrocele
Significant enlargement of one testicle should prompt further diagnostic workup to rule out a testicular tumor.
Clinical Management
Marked unilateral testicular atrophy should be reported, one reason being that it may be associated with an increased risk of testicular cancer. Imaging follow-up may be indicated, depending on the individual’s age. When imaging demonstrates unilateral testicular enlargement and malignancy cannot be ruled out with certainty, this should prompt further diagnostic evaluation.
12.2.2.4 Cryptorchidism
Cryptorchidism is the failure of one or both testes to descend into the scrotum (Table 12.9). An undescended testis may be found anywhere along its normal pathway of descent (abdominal, inguinal). Incomplete or absent testicular descent is the most common congenital anomaly in boys (2–8 %). The unilateral form is four times more common than bilateral cryptorchidism. Congenital cryptorchidism usually disappears within the first year of life (due to endogenous testosterone production), resulting in a prevalence of 1–2 % at the age of 3–12 months (Virtanen et al. 2007). An infant diagnosed with cryptorchidism should undergo orchidopexy. An undescended testicle compromises fertility and has a five times higher risk of testicular cancer (Dieckmann and Pichlmeier 2004). A retractile testis is an intermediate form with the testis tending to retract from its normal position in the scrotum.
Table 12.9
Cryptorchidism
Frequency | Prevalence in adults, approx. 1 % |
Age predilection | Congenital |
Type of lesion | Benign (fivefold higher risk of testicular cancer) |
Signs and symptoms | Asymptomatic, impaired fertility |
Differential diagnosis | Retractile testis, monorchism |
When MRI fails to demonstrate one or both testicles in the scrotum, this should prompt an evaluation for cryptorchidism. A coronal T2-weighted pulse sequence with fat saturation is well suited for this purpose, as it increases the contrast between the high-signal-intensity testicle and surrounding tissue (Fig. 12.13). An atrophic undescended testis may be more difficult to detect. A careful search along the course of the spermatic cord may help the radiologist in identifying a missing testis (Nguyen et al. 1999).
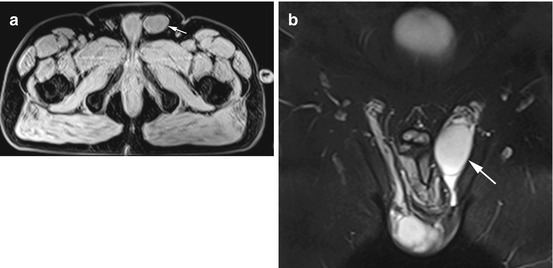

Fig. 12.13
(a) Axial T1w VIBE image reveals a mass of soft-tissue signal intensity in the left inguinal canal (arrow). (b) Coronal TIRM image with better visualization of the undescended testis in the left inguinal canal (arrow). There is fluid in the inguinal canal. Also clearly seen is the tortuous course of the spermatic cord above the testis
Clinical Management
Cryptorchidism must be communicated in all cases (although the condition is probably known). Orchiectomy is indicated in men up to 50 if no contraindications are present. In men over 50 and men at significant anesthetic risk, follow-up to rule out testicular cancer should be recommended (Wood and Elder 2009).
12.2.3 Epididymis
Disease of the epididymis is very rare (except for epididymitis). The most common lesions are cysts such as true epididymal cysts, dermoids, and spermatoceles. The most common solid epididymal masses are lipomas and adenomatoid tumors. Rarities include granuloma in men with sarcoidosis and malignant neoplasms (Woodward et al. 2003).
Clinical Management
Epididymal tumors, especially malignant ones, are very rare. Nevertheless, further diagnostic workup is indicated if MRI demonstrates solid tumor components or marked epididymal enlargement.
12.2.4 Spermatic Cord
The most common disease of the spermatic cord is a varicocele. Tumors of the spermatic cord are rare.
12.2.4.1 Varicocele
A varicocele is the dilatation of the veins of the pampiniform plexus, which may be secondary or idiopathic. A secondary varicocele results from compromised venous drainage, e.g., when a tumor is present. The more common idiopathic form has been attributed to incompetent testicular vein valves. Varicoceles are found in about 15 % of all men and in 40 % of infertile men (Table 12.10). A varicocele is considered to be present if pampiniform plexus veins are dilated to diameters of 2–3 mm (Kim and Lipshultz 1996). The markedly dilatated and tortuous veins are clearly depicted on coronal T2-weighted images with fat saturation (Fig. 12.14). The veins have intermediate signal intensity on T1-weighted images and are markedly hyperintense on T2-weighted images with the signal intensity generally being determined by the flow rate. The varicose veins enhance after contrast medium administration.
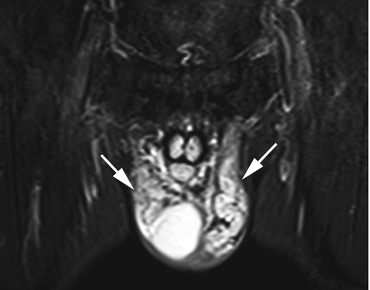
Table 12.10
Varicocele
Frequency | Prevalence, approx. 15 % |
Age predilection | Becomes more common with age |
Type of lesion | Benign |
Signs and symptoms | Usually asymptomatic, tenderness at times, impaired fertility |
Differential diagnosis | Spermatic cord tumor |

Fig. 12.14
Coronal TIRM image reveals markedly dilated and elongated pampiniform plexus veins bilaterally (arrows). Part of the right testis is also depicted in the image
A spermatic cord tumor must be ruled out in the differential diagnosis (Mattrey 1991; Cramer et al. 1991).
Clinical Management
Younger men who can be assumed to wish to have children should be informed so that treatment can be contemplated to improve fertility (Kim and Lipshultz 1996). Disclosure is not necessary in older men.
12.2.4.2 Tumors
Spermatic cord tumors are very rare. Lipomas account for half of all masses of the spermatic cord and, as elsewhere in the body, have characteristic MRI features (Beccia et al. 1976). Other benign spermatic cord tumors include leiomyoma, dermoid cysts, and lymphangioma. Once a lipoma has been ruled out on the basis of its MRI appearance, the likelihood of a malignant tumor increases to over 50 % (Beccia et al. 1976). The most common malignant tumors are sarcomas (rhabdo- and leiomyosarcoma, liposarcoma).
Clinical Management
Spermatic cord tumors are very rare, and about half of them are accounted for by lipomas. Once a lipoma has been ruled out, the likelihood of a malignant tumor is markedly higher. This situation needs to be reported for further diagnostic workup (Beccia et al. 1976).
12.2.5 Prostate
Prostate changes are very common, especially in older men. The most common finding is enlargement of the prostate, which may be due to benign prostatic hyperplasia or prostate cancer. These two entities are discussed below.
12.2.5.1 Prostate Cancer
Prostate cancer is the most common cancer in men, accounting for 25 % of all male malignancies (Table 12.11). In Germany, 58,000 men are diagnosed with prostate cancer each year. It is the third most common cause of death from cancer. The mean age at diagnosis is 69 years. However, it is assumed that over 50 % of all prostate cancers diagnosed with the PSA test would not have become apparent through symptoms during a man’s lifetime (Robert-Koch-Institut und die Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. 2008). Ninety-five percent of all prostate cancers are adenocarcinomas.
Table 12.11
Prostate cancer
Frequency | Incidence, approx. 100/100,000 men |
Age predilection | Mean age at presentation, 69 years |
Risk factors | Androgen dependency, obesity, smoking, genetic predisposition |
Type of lesion | Malignant |
Signs and symptoms | Disturbed bladder voiding, back pain |
Differential diagnosis | Benign prostatic hyperplasia |
The zonal anatomy of the prostate is helpful for interpreting and reporting MRI findings.
Four zones are distinguished:
The peripheral zone, which accounts for 70 % of the total prostate volume and is the most common site of prostate cancer (70 %)
The central zone, which accounts for 25 % of the prostate and harbors 5 % of prostate cancers
The transitional zone, where 20 % of cancers are found
The normal prostate gland has homogeneous intermediate signal intensity on T1-weighted images, which do not depict the zonal anatomy and provide poor soft-tissue contrast for identification of pathology. T2-weighted images are more useful, allowing differentiation of the zonal anatomy. The peripheral zone has high signal intensity and is surrounded by a black line, which corresponds to the prostate capsule. The central and transitional zones are of inhomogeneous low signal intensity due to the high proportion of stroma. Prostate cancer usually has low T2 signal intensity (and may be multifocal), which is why it is easier to detect in the hyperintense peripheral zone than in the remaining prostate (Fuchsjager et al. 2008; Turkbey et al. 2009). DWI may show restricted diffusion with an increased signal intensity and low ADC values (Issa 2002). Common accessory findings include infiltration of the bladder base, invasion of surrounding fatty tissue, locoregional lymph node metastases, and (osteoblastic) bone metastases.
The differential diagnosis includes benign prostatic hyperplasia (typically developing in the transitional zone), hemorrhage, prostatitis, and bladder cancer extending into the prostate (Fuchsjager et al. 2008; Chang et al. 2008). It is likely that, with the protocol used for a whole-body screening examination, many prostate cancers are overlooked (thick slices, surface coils).
Clinical Management
Suspected prostate cancer should be disclosed, regardless of the man’s age, and further diagnostic workup recommended (PSA test). Inconclusive findings, which are not uncommon, should also be reported as a variety of specific diagnostic options are available (Fuchsjager et al. 2008).
12.2.5.2 Benign Prostatic Hyperplasia
Benign prostatic hyperplasia (BPH) is nonmalignant enlargement of the prostate gland resulting from a nodular increase in volume of the transitional zone due to cellular hyperplasia. The prevalence increases with age (Table 12.12). An autopsy study showed a prevalence of 40 % at age 50 and of 70 % at age 60 with 90 % of men in the ninth decade of life having signs of BPH (Berry et al. 1984). The predominant symptoms are due to obstruction and irritation. One method of diagnosing BPH is measurement of prostate volume. However, changes in prostate volume over time are subject to wide interindividual variation. While hyperplasia is common, a considerable proportion of aging men have a stable or decreasing prostate size (Loeb et al. 2009). The only feature seen on T1-weighted images is enlargement, while T2-weighted pulse sequences also show that the enlarged transitional zone is very heterogeneous and of low signal intensity. Often, the enlarged gland will elevate the bladder base. In advanced BPH, MRI depicts signs of urinary obstruction with a large bladder volume or evidence of a trabeculated bladder. Ultimately, however, BPH cannot be confidently differentiated from prostate cancer in the screening situation (Fig. 12.15) (Fuchsjager et al. 2008).
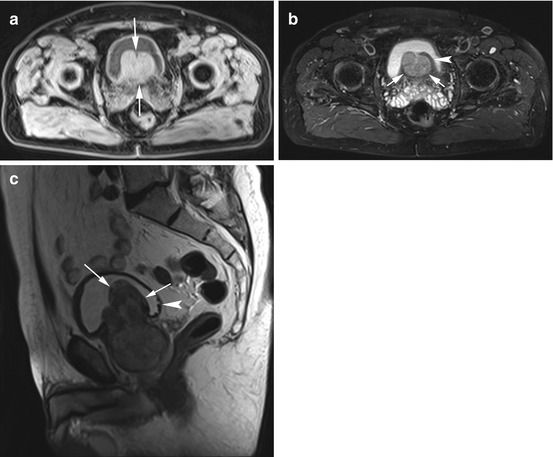
Table 12.12
Benign prostatic hyperplasia
Frequency | Prevalence in 60-year-old men, 70 % |
Age predilection | BPH becomes more common with age |
Risk factors | Androgen dependency, age |
Type of lesion | Benign |
Signs and symptoms | Difficult bladder voiding |
Differential diagnosis | Prostate cancer |

Fig. 12.15
(a) Axial T1w VIBE image reveals a polypous mass in the urinary bladder (arrows). The mass appears homogeneous and isointense to soft tissue. (b) PD image also demonstrates the slightly inhomogeneous and smoothly marginated mass (arrows). Portions of the bladder wall are intact (arrowhead), suggesting that the mass arises outside the bladder. Both seminal vesicles are somewhat enlarged. (c) Sagittal T2w image confirms a polypous mass of the prostate with massive extension into the bladder lumen (arrows). The mass is predominantly hypointense with a zone of inhomogeneous signal intensity and has a volume of about 100 cm3. There is evidence of urinary obstruction, and early signs of trabeculation are apparent in the posterior wall (arrowhead)
Clinical Management
We recommend performing volumetry with measurement of all three diameters for calculating the prostate volume according to the following formula: V = 1/6 Π abc. Prostate enlargement should be reported when the calculated volume is greater than 45 cm3 or when signs of urinary obstruction are present.
12.2.6 Seminal Vesicles
The seminal vesicles are paired glands located posterior to the urinary bladder and distal to the ureters. They are approx. 3 cm long and 1.5 cm wide, and their size first increases with age to then decrease again in very old age. Differences in size between the two seminal vesicles are not uncommon (Aboul-Azm 1979). There may be congenital unilateral or bilateral agenesis of the seminal vesicles or cystic degeneration (Arora et al. 2007). The latter is frequently associated with other congenital anomalies of the urogenital tract (Chen et al. 2006




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree