Antithyroid drug
Surgery
Radioiodine
Time to initial improvement
2–4 weeks
Needs antithyroid drug pretreatment (toxic goiters)
Needs antithyroid drug pretreatment; 4–8 weeks after RIT
Recurrence
90–100 % (toxic goiters)
1–4 %
5–10 %
Use in pregnancy/Breast feeding
Yes (First trimester: PTU preferred)
Second trimester
No
Adverse effect on eye disease
No
No
0.1 % (1 % risk of immune hyperthyroidism; rarely eye symptoms)
Interference with daily activities
No
Requires hospital admission
Need for radiation precautions
Key adverse effects
Rash; arthralgia; hepatitis; agranulocytosis
Surgical; vocal cord paralysis; hypoparathyroidism; hypothyroidism
Hypothyroidism
Surgery is preferably performed in selected cases of nodular goiter, when there is additionally a suspicion of malignancy, when there is a severe compression of neighbouring structures, or when an immediate effectiveness of therapy is necessary due to severe adverse effects of ATDs.
Radioiodine is in most cases the first-line treatment for solitary hyperfunctioning thyroid nodules and toxic multinodular goiter without hypofunctioning nodules or large cystic degenerations. The main indications for radioiodine treatment of NTG are to reduce the size of a goiter that is causing cosmetic difficulties for the patient and to relieve compressive signs or symptoms. The available treatment options in NTG patients in whom the risk of malignancy is considered low are “wait and see” policy, surgery, levothyroxine (LT4, in a non-TSH-suppressive dosage) and radioiodine administration (Table 2). In patients with postoperative goiter recurrence, radioiodine therapy is often considered first-line therapy and the physician should not wait until the patient becomes symptomatic. The optimal timing of radioiodine therapy is important for patients with pre-existing paralysis of the N. recurrens (with or without previous thyroid surgery), especially if paralysis is contralateral to a unilateral recurrent goiter, and for patients with temporary postoperative hypoparathyroidism after the first operation. With levothyroxine medication some patients may achieve clinically relevant (>50 %) nodule or goiter shrinkage, however, it occurs only in 10–20 % of patients (Fast et al. 2008). The only available controlled long-term investigation (Papini et al. 1998) showed no significant nodule size reduction after 5 years of continuous LT4-therapy. Additionally, a comparative study showed the superiority of I-131 treatment over LT4-therapy (Wesche et al. 2001). Radioiodine treatment is indicated in patients with medical contraindications to thyroid surgery, patients with slight or moderate compressive symptoms, patients with large goiter, or patients who wish to avoid operation. The interdisciplinary approach to patients followed by well-balanced decision making and informed consent allow for an individualized selection between alternative treatment options. Special consideration should be given to the patient’s profession as I-131 is without risk for paralysis of the recurrent laryngeal nerve. There are well-known regional differences in the choice of treatment for initial or recurrent hyperthyroidism, but radioiodine is increasingly used as first-line treatment as ever more data accumulate proving its safety.
Table 2
Main features of the three available treatments for non-toxic goiter [modified from Weetman (2007)]
Levothyroxine | Surgery | Radioiodine | |
|---|---|---|---|
Efficacy | Poor | Excellent | 50 % reduction in 1st year, 70 % reduction after 4 years |
Relief of symptoms | Slow | Immediate | Medium term |
Recurrence | If treatment stopped | 1–40 % (depend on size of thyroid remnant) | Unlikely; repeat treatment feasible |
Special indications | Small goiter; patient wish | Suspicion of malignancy; severe compression of neighbouring structures | Patient wish; elderly with surgical risk factors; postoperative goiter recurrence |
Exclusions | Suppressed TSH | Surgical contraindications | Radiation protection issues |
Key adverse effects | Depending on TSH-level: cardiac arrhythmia, osteopenia | Surgical; vocal cord paralysis; hypoparathyroidism; hypothyroidism | Hypothyroidism |
4 Physical and Radiobiological Properties of Radioiodine
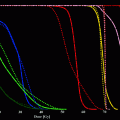
Iodine is an indispensable component of thyroid hormones levothyroxine (T4) and triiodothyronine (T3). Thyroid cells extract and concentrate iodide from plasma. Shortly after administration, radioiodine is taken up from the blood via the sodium–iodine symporter and accumulates within thyroid follicular cells (Fig. 1). About 20 % of the iodide perfusing the thyroid is removed at each passage through the gland. Ingested iodine is absorbed through the small intestine and transported in the plasma to the thyroid, where it is concentrated, oxidized, and then incorporated into thyroglobulin (Tg) and later T4 and T3. After storage in thyroid follicles, Tg is subjected to proteolysis and the released hormones are secreted into the circulation. In subjects with normal thyroid function up to 20–30 % of orally administered iodine is taken up by the thyroid. In hyperthyroid patients this fraction is increased—in individual cases up to >80 % (rationale for pretherapeutic radioiodine testing). The fraction of radioiodine which is not taken up by the thyroid gland is mainly excreted via the urine (Fig. 2).
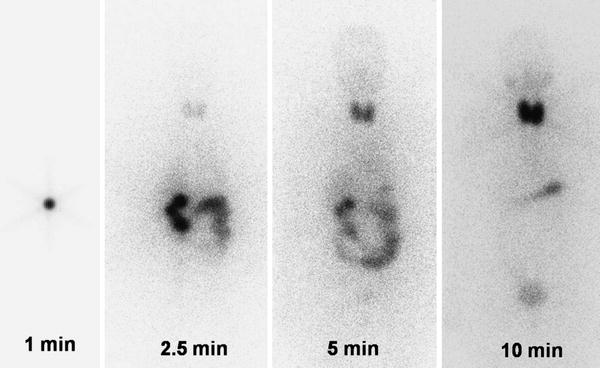
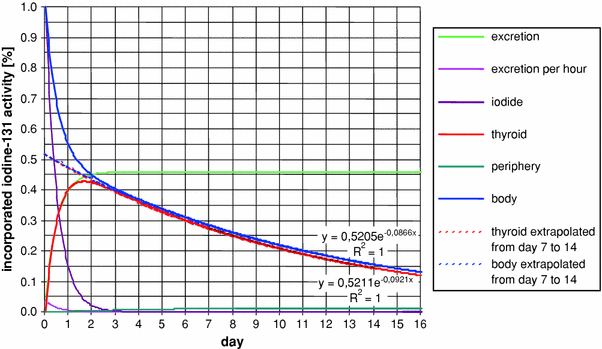

Fig. 1
Example for the rapid I-131 thyroidal uptake demonstrated by dynamic whole-body images: 1 min after the application of an I-131 therapy capsule the activity is visible in the stomach, after 2.5 min activity in the gut, after 5 min increasing thyroidal accumulation, and after 10 min the thyroidal accumulation is dominating

Fig. 2
Modeling of iodine kinetics
Iodine-131 (I-131) is a beta- and gamma-emitting radionuclide (principle β– = 0.807 MeV; gamma rays ranging from 80 to 637 keV, most abundant γ: 364 keV) with a physical half-life of 8.02 days. The average range of the 0.807 MeV beta particles in soft tissue approximates 1 mm which allows high intrathyroidal doses. Radiobiological effects of radioiodine on tissues are direct (radiation deposit within DNA) or indirect. Indirect effects produce free radicals that in turn react with the critical macromolecules. I-131 used for treatment of thyroid disorders decays to stable Xe-131 by beta emission.
The radiopharmaceutical I-131 sodium iodide is administered orally in capsules, but in patients with severe swallowing difficulties it can be administered in liquid or intravenously in patients in whom vomiting is a problem.
5 Contraindication for Radioiodine Therapy and Precautions
5.1 Pregnancy, Breast Feeding
Pregnancy and breast feeding are all absolute contraindications to radioiodine treatment. Most recommendations stipulate that pregnancy should be avoided for 4 months after radioiodine treatment in women and in men (Dietlein et al. 2007b; Cooper et al. 2009). Arguments for this waiting period are radiation protection and stability of thyroid function in women, the cycle of spermatogenesis in men. This in turn implies that the clinician must have a careful discussion with each patient for whom there may be plans for conception, and treatment must be planned with this in mind. More generally, each patient requires a careful explanation of all treatment options provided by a specialist accredited in the treatment of thyroid disease.
5.2 Uncontrolled Hyperthyroidism
Since radioiodine administration may cause a transient elevation in free T4 and free T3 levels approximately 7 days following administration, uncontrolled symptoms of hyperthyroidism or high level of free T3 constitute relative contraindications for therapy (further elevation of thyroid hormone may trigger atrial fibrillation or heart failure or, rarely, lead to thyroid storm). In that case, pretreatment with antithyroid drugs combined, if necessary, with β-blockers should be administered first. In the symptomatically well-controlled patient radioiodine therapy will have little effect on patient clinical symptoms. In highly selected cases where antithyroid drug are contraindicated (e.g., due to agranulocytosis or post-therapy liver failure) and surgery cannot be performed due to symptoms of hyperthyroidism, I-131 may be administered under the cover of steroids (usually hydrocortisone hemisuccinate 50–100 mg i.v.) and β-blockers.
5.3 Tracheal Stenosis
Patients with large goiter with tracheal narrowing <1 cm may be treated under the cover of steroids though as per authors’ experience clinically harmful thyroid swelling was never observed with tracheal diameters ≥5–6 mm. If tracheal diameter is <5–6 mm, due to risk of severe dyspnea surgery rather than radioiodine therapy should be performed. Patients with high risk of severe post-treatment complications (elderly with a risk of heart failure, patients with narrow tracheal diameter, large thyroid goiter or with uncompensated hyperthyroidism) should always be treated in inpatients facilities to provide continuous medical surveillance.
5.4 Hypersensitivity
Radioiodine administration is very unlikely to precipitate a hypersensitivity reaction because I-131 free of large stable iodine contamination is administered, so even in cases of known iodine sensitivity the I-131 therapy can be safely performed. The stable iodine content of radioiodine preparations is 0.05–0.18 μg. This is very significantly lower than average daily iodine intake.
6 Procedure
6.1 Facility and Legislation
The facilities required to perform radioiodine therapy will depend on the national legislation for the emission of pure beta- or beta–gamma-emitting therapy agents. The requirement to admit patients due to administered I-131 activity varies considerably across Europe. If in-patient therapy is required by national legislation, this should take place in an approved environment with appropriately shielded rooms. The facility in which treatment is performed must have appropriate personnel, radiation safety equipment, procedures available for waste handling and disposal, and handling of incidental contamination, monitoring personnel for accidental contamination and controlling/limiting its spread.
The restrictions on work and contact with small children depend on national dose limits. Generally in Europe, the exposition from radioiodine radiation should not exceed 1 mSv for other individuals in the general population. Usually, the patient is advised to keep as much distance as possible between themselves and others, including children, and of keeping contact times as short as possible (ICRP 2004; Koch et al. 2005; Cappelen et al. 2006; Dillehay et al. 2006).
6.2 Patient Preparation
Patient evaluation before radioiodine therapy should include:
1.
Patient’s history with special emphasis on previous treatments (e.g., use of antithyroid drugs, iodinated contrast media, amiodarone, other iodine containing medication, iodinated multi-vitamin combinations) and urinary incontinence. Exposure to excessive stable iodine may influence the choice of primary treatment and the timing of radioiodine therapy. After the administration of iodinated water-soluble contrast agents, radioiodine therapy should be postponed for about 6–8 weeks. In cases of amiodarone induced thyrotoxicosis the elimination of the excess iodine load can take up to 1–2 years (on average 6 months) in amiodarone induced thyrotoxicosis (Eskes and Wiersinga 2009). In doubtful cases, measurement of iodine excretion in the urine is possible. If amiodarone cannot be stopped because of the underlying heart problem, then radioiodine treatment will not be feasible. In patients presenting with unmanageable urinary incontinence a lifelong medication with ATDs may be justified in case of overt hyperthyroidism. TSH-level should be kept in the lower range to avoid increase of the thyroid volume. Consequently, information on this is important and should be part of the patients’ evaluation before therapy.
2.
Laboratory testing, including free T4, free T3, TSH, TPO-Ab, and Tg-Ab.
3.
Thyroid scintigraphy and radioiodine testing with radioiodine 24-h uptake: The 24-h uptake should be >20 %, if lower other treatment modalities should be considered. Uptake measurements are not absolutely required when fixed activities are used.
4.
5.
Fine needle aspiration biopsy (FNAB) in nodules larger than 1–1.5 cm with a suspicious sonographic appearance or scintigraphically hypofunctioning. In elderly patients with large goiters, the combination of hyperfunctioning nodules (indication for radioiodine therapy) and hypofunctioning nodules (surgery as preferred option) is a common constellation. An individual risk stratification is necessary. In autonomous nodules, as the risk of malignancy is very low, FNAB should only be considered in case of suspicious sonographic features (Cooper et al. 2009). Alternatively, I-123 scintigraphy with late imaging 24 h p.i. is a strategy to confirm autonomously functioning nodules and to exclude Tc-99m pertechnetate “trapping only” nodules.
6.
Female patients with child bearing potential should be routinely tested for pregnancy within 72 h or less before the administration of I-131. When patient history clearly indicates that pregnancy is excluded, a pregnancy test may be omitted at the discretion of the treating physician. Consequently, post-therapeutic contraception for 4 months after I-131 therapy is also a prerequisite.
6.3 Special Considerations for Medication
Antithyroid drugs are often used in the initial treatment of patients with hyperthyroidism. As pretreatment with ATDs depletes thyroid hormone stores, it constitutes a safe patient preparation for radioiodine treatment, especially beneficial in patients with overt hyperthyroidism or distinctly elevated fT3. However, since thyrostatic drugs may lower the uptake of radioiodine as well the effective half-life they can decrease effectiveness of radioiodine treatment. Another side-effect of ATDs is the possible radioprotective effect which seems to depend on chemical compounds contained in the thyrostatic medication (methimazole and carbimazole, which do not possess sulfhydryl groups probably do not have radioprotective effects) (Moka et al. 2002). The potentially negative impact of thyrostatic drugs can be compensated for by discontinuing medication shortly before treatment; carbimazole and methimazole should be withdrawn respectively for at least 2 days before planned radioiodine administration (Braga et al. 2002; Bonnema et al. 2004; Santos et al. 2004) if tolerated by the patient. PTU which has a more distinct radioprotective action that may further reduce the effectiveness of radioiodine should be stopped at least 2–3 weeks before radioiodine treatment. When data from an individual pretherapeutic dosimetry (radioiodine testing with measurement of the effective half-life) are available, a withdrawal of PTU 2 days before “radioiodine testing” and radioiodine therapy may be sufficient (Kobe et al. 2008a). Beta adrenergic antagonists (usually propranolol in dose adjusted to clinical symptoms) may be helpful for interim hyperthyroidism symptom relief during ATD withdrawal, provided that there are no contraindications.
Levothyroxine medication decreases radioiodine uptake, but may be used to optimized the distribution of radioiodine within the nodular thyroid. In patients with autonomously functioning nodules and a pretherapeutic TSH-level of >0.3 mUL−1, the levothyroxine medication lead to an exogenous suppression of the thyrotropin secretion and may be favorable to achieve euthyroidism without levothyroxine substitution. If reduction of goiter size is given priority, TSH suppression is not necessary.
Lithium can block radioiodine release from the thyroid but does not interfere with radioiodine uptake. It may enhance the effectiveness of radioiodine when given for a few days immediately after treatment, but the available data are controversial and side-effects may be experienced by 10 % of patients. In one prospective, randomized, controlled trial from Italy, lithium treatment (900 mg/day for 6 days from the time of radioiodine treatment) increased the cure rate by 11 % and induced a more rapid control of hyperthyroidism (Bogazzi et al. 1999). However, another randomized, controlled trial from India has found no evidence of an effect of lithium on outcome after radioiodine treatment (Bal et al. 2002). Therefore, the off-label use of lithium (e.g., 2 × 250 mg) is not routinely recommended. After recompensation of thyroid function in toxic goiters, there is usually no need for a prolongation of the effective half-life.
Recombinant human TSH (rhTSH) may be used to maximize I-131 uptake in the thyroid gland and to minimize the radiation dose to the remainder of the body in patients with non-toxic multinodular goiter (Silva et al. 2004; Nielsen et al. 2006a, b; Fast et al. 2009). However, rhTSH is not yet approved for this indication and administration in a patient with non-toxic goiter represents an off-label use.
7 Patient Information and Instruction
Patients should receive both written and verbal information about the procedure before receiving therapy. Written informed consent must be obtained from the patient. The following items should be discussed:
Therapeutic options and alternatives including watchful waiting.
More than one I-131 treatment course may be necessary. Pretherapeutic dosimetry before I-131 therapy and intended absorbed doses of about 100–150 Gy (toxic goiters, non-toxic goiters) and 300–400 Gy (toxic nodules) offer high rates of success of a single I-131 therapy.
Recommendations for radiation protection after discharge from hospital or as out-patient.
Early and late side-effects.
Need for contraception for 4 months for female and male patients.
Need for lifelong follow-up.
8 Radiation Dosimetry and I-131 Activity
The aim for radioiodine treatment in toxic nodules or toxic goiters is often to restore euthyroidism (Dietlein et al. 2007b). There have been many studies attempting to identify an optimal regimen for radioiodine treatment that minimizes the risks of developing hypothyroidism while maximizing the cure rate of hyperthyroidism. However, this is a challenging issue: first because a maximum cure rate inevitably results in the highest rate of hypothyroidism and second because any single dosage method cannot practically encompass all of the variables affecting outcome. These variables include age, sex, thyroid size, severity of hyperthyroidism, innate differences in radiation sensitivity, iodine intake, and preceding use of antithyroid drugs. Nevertheless, there is an ongoing discussion on the establishment of the optimal method to determine the activity that can be recommended for clinical practice: estimation (the so-called “fixed dose”) versus calculation (based on radioiodine uptake measurements). Council directive Euratom 97/43 requires that for all medical exposure of individuals for radiotherapeutic purposes exposures of target volumes will be individually planned. In principle, formulas which calculate the therapeutic I-131 activity for an individual patient differ in the time points when radioiodine uptake is measured during the radioiodine test. The Marinelli formula takes a 24-h radioiodine uptake while Hänscheid–Bockisch formula focuses on a late uptake about 5 days after ingestion of a test capsule (Marinelli et al. 1950; Bockisch et al. 1993).
In either toxic or non-toxic multinodular goiter, radioiodine doses have been empirically established. The optimal radioiodine therapy should be a single dose cure. Currently, an absorbed radiation dose of 100–150 Gy is recommended (Table 3), requiring about 3.7–5.5 MBq per gram of thyroid tissue corrected for the 24-h I-131 uptake (Hegedus et al. 2003). To increase radioiodine uptake and retention in non-toxic goiter, rhTSH was used in some studies (Braga et al. 2002; Bonnema et al. 2004). rhTSH can increase the absorbed radiation dose and permits a more equal distribution in the gland (Nieuwlaat et al. 2003; Silva et al. 2004; Nielsen et al. 2006a, b). Its future role, however, still has to be established. In patients with autonomous nodules, the recommended dose is 300–400 Gy (Fig. 3).
Table 3




Recommendations for intended target dose
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree