Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer
Post-surgical use of radioiodine (I-131) in patients with papillary and follicular thyroid cancer and the issue of remnant ablation: A consensus report
European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium
Guidelines for radioiodine therapy of differentiated thyroid cancer
Thyroid cancer: ESMO clinical guidelines for diagnosis, treatment and follow-up
From a practical viewpoint, the risk classification of patients with DTC according to prognostic indicators is of great clinical relevance when choosing treatment strategies for a given individual. Here, the classification of the European Thyroid Association (ETA) seems to be useful (Table 2).
Table 2
Differentiated thyroid cancer (DTC): risk classification, adapted from Reiners et al. ( 2008a)
Organization | Risk classification characteristics | |||
|---|---|---|---|---|
UICC, AJCC, ATA Stage I: Stage II: Stage III: Stage IV: | Pts. <45 years Any T or N, M0 Any T or N, M1 Not applicable Not applicable | Pts. ≥45 years T1, N0, M0 T2, N0, M0 T3N0M0 or T1/T2N1M0 all other TNM categories | ||
ETA | Very low risk T1(≤ 1 cm)N0M0 | Low risk T1(>1 cm)N0M0 T1mN0M0 T2N0M0 | High risk T3 and T4 N1 M1 | |
3 Therapeutic Strategy Therapeutic Strategy
The aims of radioiodine ablation of postoperative thyroid remnants are:
prevention of locoregional recurrences,
reduction of mortality,
detection of previously unknown metastases by post-ablation 131I whole-body scintigraphy (WBS),
improvement of follow-up using 131I WBS, stimulated Tg measurements, or both.
Comprehensive retrospective long-term studies (Sawka et al. 2004, 2008a) with more than 10 years of follow-up showed that ablative radioiodine therapy decreased the rates of locoregional recurrence and disease-associated mortality (Table 3).
Table 3
Efficacy of ablative radioiodine treatment in long-term follow-up, adapted from Sawka et al. (2004)
Institution | Patients (n) | Follow-up (years) | Statistical significance of improvement in rates of: | |
|---|---|---|---|---|
Mortality | Recurrences | |||
Ohio state | 1,510 | 16.6 | P < 0.00001 | P < 0.016 |
Pisa | 964 | 12 | n.s. | P < 0.001 |
MD Anderson | 1,599 | 11 | P < 0.001 | |
Toronto | 382 | 10.8 | n.s. | |
UCSF | 187 | 10.6 | n.s. | P < 0.0001 |
Hongkong | 135 | 9.2 | n.s. | |
Gustave Roussy | 273 | 7.3 | n.s. | |
Gundersen | 177 | 7.2 | n.s. | |
Illinois Registry | 2,282 | 6.5 | n.s. | |
Mexico | 229 | 5 | n.s. | |
Radioiodine ablation is indicated in the high-risk group (pT3, pT4, each N1, each M1) as well as in the low-risk group (pT1b, pT2), if the tumor is multifocal (pT1 m) and if lymphnode metastases and/or distant metastases are present (Pacini et al. 2005; Luster et al. 2008; Dietlein et al. 2007a).
Since metastases cannot be excluded safely by low post-operative thyroglobulin levels <2 ng/ml, such low levels of the thyroid-specific tumor marker can not be used as an argument to refrain from radioiodine ablation. In a recent study, Park et al. (2009) showed that iodine avid metastases were present in 6 % of patients (52/824) with post-operatively low thyroglobulin levels <2 ng/ml (without interference of Tg-antibodies).
4 Very Low Risk Patients Very Low Risk Patients
Patients with unifocal, papillary carcinomas ≤1 cm in diameter without unfavorable histological characteristics, such as a tall cell or diffuse sclerosing variant, belong to the very-low risk group of ETA-Classification (Pacini et al. 2005, 2006a). In these individuals, radioiodine ablation is not necessary since total thyroidectomy is neither indicated nor performed regularly and thyroid remnants tend to be too large for safe destruction by 131I. Although some retrospective follow-up studies showed a reduction of the locoregional recurrence rate in this setting after adjuvant ablative radioiodine therapy (Pelizzo et al. 2004), the procedure seems not to be justified in the very-low-risk group, given the virtually 100 % disease-free survival in its absence. Nonetheless, the probability of lymphogenic micrometastases significantly increases in papillary thyroid cancer above a tumor diameter of 5 mm (Machens et al. 2005; Verburg et al. 2008). After near-total thyroidectomy, adjuvant ablative radioiodine therapy improves the follow-up and may be discussed with the patient (Dietlein et al. 2007a) in accordance with other prognostic factors (tumor close to the thyroid capsule, previous percutaneous radiation to the neck, familial occurrence of thyroid cancer, tumor diameter between 5 and 10 mm and unfavorable DTC histological variants cancer). Similar concerns may be taken into account for small follicular thyroid cancers with a diameter less than 1 cm, which are however rarely encountered (Machens et al. 2005; Rahbar et al. 2008).
5 Procedure for Radioiodine Ablation Procedure for Radioiodine Ablation
5.1 Pre-therapeutic Scintigraphy
The application of a diagnostic activity of 131I immediately before radioiodine treatment may induce “stunning,” that is, reduced uptake or altered kinetics of 131I during subsequent radioiodine therapy, which may substantially impair the efficacy of that therapy and reduce the rate of successful ablation by as much as 50 % (Lassmann et al. 2004; Lundh et al. 2007, 2009; Verburg et al. 2009). Therefore, it is recommended not to use the diagnostic activities of 131I that are necessary for scintigraphy (more than 10–20 MBq) before ablation (Medvedec 2005). As an alternative, 24-h uptake measurements with smaller 131I activities (<10 MBq) may be performed to roughly estimate the mass of the thyroid remnant (Dietlein et al. 2007a, b). As an additional alternative to 131I diagnostic scanning, iodine-123 (123I) WBS may be used. Because 123I is a pure gamma emitter, it has the advantage of causing no stunning (Lundh et al. 2009). However, given its 13.2 h half-life, this relatively expensive radionuclide does not allow late scans (i.e., >24 h after 123I administration) with low background activity. 123I scintigraphy therefore is regarded less sensitive than 131I scanning. If 123I is used for WBS, a 40–200 MBq activity must be applied.
Recently, positron emission tomography (PET) with 40–100 MBq of the radioisotope 124I (half-life 4.2 days) has been tested as still another alternative for localization of thyroid remnants or for pretherapeutic dosimetry (Freudenberg et al. 2007). However, no systematic data are available up to now with respect to stunning, which at least hypothetically may be induced by 124I, a positron—as well as a gamma—and beta-emitter.
5.2 TSH Stimulation with Recombinant Human TSH or Thyroid Hormone Withdrawal
Ablative radioiodine treatment demands serum TSH levels above 30 mU/l to induce sufficient radioiodine uptake in thyroid remnants or tumor tissue. Such TSH stimulation may be achieved by two different approaches:
When withholding levothyroxine replacement, some authors recommend giving patients shortlived triiodothyronine (T3, pharmacological half-life approximately 10 h) for one or—at most—2 weeks immediately after surgery or—when thyroid hormone withdrawal (THW) is undertaken later in the post-surgical course, after levothyroxine (T4, pharmacological half-life approximately 10 days) has been discontinued.
A randomized prospective Phase III study in mainly low-risk patients proved that a standard activity of 3.7 GBq 131I induced comparable ablation rates of approximately 90 % after endogenous (TSH >25 mU/l after THW) or after exogenous TSH-stimulation (two consecutive daily i.m. injections of rhTSH, 0.9 mg). These rates were obtained when successful ablation was defined as the combination of radioiodine neck uptake <0.1 % on a diagnostic WBS and of serum Tg < 2 ng/ml under rhTSH stimulation at 8 ± 1 months after ablation (Pacini et al. 2006b). In the meantime, the long-term follow-up of patients included in this prospective phase-III trial proved the equal efficacy of radioiodine ablation prepared by recombinant TSH as compared for thyroid hormone withdrawal (Elisei et al. 2009). On the other hand, a nearly 400-patient retrospective analysis from the Memorial Sloan-Kettering Cancer Center (Tuttle et al. 2008a), which also used large activities of 131I and included a higher risk study population than the Phase III trial, found that, with a median 2.5 years of follow-up, rhTSH- and withdrawal-aided ablation were associated with statistically not different, low rates of clinical recurrence or persistent thyroid bed scintigraphic uptake, as well as statistically similar time to recurrence. This led the American Thyroid Association (Cooper et al. 2009), the European Society for Medical Oncology (Pacini et al. 2010) and the European Association for Nuclear Medicine (Luster et al. 2008) to recommend preparation of radioiodine ablation with recombinant TSH because of its equal effectivity but lower rate of side-effects. In 2009, the European Medicine Agency (EMEA) expanded the indication for rhTSH in combination with 3.7 GBq 131I from “low risk (pT1-2, N0, M0) patients” to patients after near-total or total thyroidectomy of differentiated thyroid cancer tumor stages pT 1–4, N 0–1, M0.
Recently, Tuttle et al. from the Memorial Sloan Cancer Institute, New York showed in a retrospective study that radioiodine had similar therapeutic (tumoricidal) effects on small volume I-131 with metastatic disease in both locoregional lymph nodes and pulmonary parenchyma (Tuttle et al. 2010).
Advantages of ablative radioiodine treatment under recombinant TSH were avoidance of hypothyroid morbidity and hence, improved quality of life, and lower radiation exposure of extrathyroidal compartments due to lesser (−35 % on average) radioactivity in the blood. The latter finding can be explained by non-disturbed renal clearance of radioiodine in the euthyroid state (Hanscheid et al. 2006; Luster et al. 2003; Loffler et al. 2003). As a consequence of decreased whole-body radiation exposure, a lower risk for secondary cancers after radioiodine therapy (see below) must be taken into account, at least hypothetically.
According to model calculations from a societal perspective by Mernagh et al. (2006), the use of rhTSH rather than THW led to a gain of 0.05 quality adjusted life years (QALYs) per course, which was caused by shorter missed worktime and an extrapolated lower rate of secondary neoplasia. The additional cost associated with this increase in QALY, calculated as an incremental cost-effectiveness ratio (ICER), amounted to 958 euros per QALY, which suggests that preparation of ablative radioiodine therapy by rhTSH is cost-efficient, given the generally accepted cost-effectiveness threshold, an ICER of 45,000 euros/QALY, for new interventions. In addition, short-term hypothyroidism secondary to T4 withholding after thyroidectomy leads to disturbances of lipid metabolism and increased arteriosclerotic risk (Duntas and Wartofsky 2007).
Some authors recommend performing a “mini-withdrawal” of levothyroxine a few days before the two rhTSH injections, and a few days after radioiodine administration, to reduce the supply of stable iodine provided by thyroid hormone medication (100 μg of levothyroxine contain approximately 65 μg of iodide). A study by Barbaro et al. (2003) compared urinary iodine excretion after rhTSH stimulation and continued T4 medication versus after rhTSH stimulation and thyroid hormone “mini-withdrawal.” Iodine excretion was significantly lower after “mini-withdrawal” (47.2 ± 4.0 μg/l versus 76.4 ± 9.3 μg/l, P = 0.019). The ablation success rate after 1 year was even slightly higher after rhTSH-aided ablation with short-term thyroid hormone withdrawal as compared to stimulation with traditional THW (81.2 % versus 75.0 %). In contrast, Tala Jury et al. were unable to find a relationship between urinary iodine excretion and the success rate of rhTSH-aided ablation (Tala Jury et al. 2010).
5.3 Radioiodine Activities for Remnant Ablation
If the remnant dose (in Gy) cannot be projected and an individualized activity therefore cannot be calculated for a given patient, standard fixed 131I activities between 1 and 3.7 GBq are recommended for THW-aided ablation, whereas a standard fixed activity of 3.7 GBq has been used for rhTSH-aided ablation. Within the “bandwidth” of 1 and 3.7 GBq 131I, higher activities seem to coincide with higher success rates (Hackshaw et al. 2007). A recent prospective, randomized, controlled investigation (Pilli et al. 2007) suggests that rhTSH-aided ablation with 1.85 GBq of 131I may achieve a statistically non-inferior ablation success rate to that seen with rhTSH-aided ablation using 3.7 GBq, even in the presence of node metastases. Two prospective trials (Barbaro et al. 2003, 2006), but not another (Pacini et al. 2002), suggest equivalent ablation success rates with rhTSH-aided versus withdrawal aided ablation using 1.1 GBq; the latter study by Pacini is best disregarded for the comparison between rhTSH and levothyroxin withdrawal as the I-131 activity was administered at a later than recommended timepoint after rhTSH administration. Further studies are desirable to increase the duration of follow-up that has been reported for rhTSH-aided ablation.
The amounts of radioactivity for successful ablation strongly depend on the completeness of previous surgery. Especially in countries such as Germany, where approximately 50 % of DTC patients are diagnosed “by chance” coincidentally after routine thyroid surgery for endemic nodular goiter, thyroid remnants usually are relatively large, so that even treatment activities of 3.7 GBq of 131I may not be sufficient for complete ablation of the remnant. If the 24-h uptake of a (low) diagnostic activity of 131I exceeds 20 %, the patient should be remitted to the surgeon for complete thyroidectomy. In the case of R1 resections, aggressive DTC subtypes or other high-risk features, even higher standard therapeutic activities of up to 7.4 GBq 131I can be administered without special precautions.
5.4 Individual Dosimetry
If radioiodine activities above 7 GBq are considered for ablative treatment, dosimetric approaches should be used to estimate the radiation dose to the blood, to ensure that this dose does not exceed 2 Gy, the traditionally accepted safety threshold to avoid serious myelotoxicity (Hanscheid et al. 2006; Benua et al. 1962; Lassmann et al. 2008; Flux et al. 2010).
At least in the situation of ablation therapy it is most often not possible to calculate target doses to the remnant using the MIRD formalism because of the difficulty to determine the remnant volume after surgery (Salvatori and Luster 2010). On the other hand, even in patients with advanced disease the relatively simple method to limit the radiation dose to the blood proved to be safe and effective (Verburg et al. 2010).
5.5 Low Iodine Diet
It is strongly recommended to avoid iodine-containing drugs (e.g., X-ray contrast media, reagents for disinfection, ophthalmic agents, amiodarone, iodide medication), food or food additives with high iodine content (e.g., seaweed, kelp multivitamin and trace element combinations) in the weeks preceding radioiodine ablation. A low-iodine diet with <50 μg iodine per day no seafood or iodized salt is recommended for 2–3 weeks (Cooper et al. 2009; Pacini et al. 2005; Maxon et al. 1983) before radioiodine treatment to improve radioactive iodine uptake in thyroid remnants or metastases.
6 Post-therapeutic 131I Scintigraphy Post-therapeutic 131I Scintigraphy
Performing WBS 4–6 days or even earlier in some cases with exogenous TSH stimulation after administration of therapeutic activities of 131I is mandatory for definitive staging of DTC (Fig. 1), high therapeutic activities of 131I being more sensitive than diagnostic activities, which are usually 20–30 times lower. Tomographic procedures, namely single-photon-emission computer tomography (SPECT) can improve the sensitivity of post-therapy scans as compared to conventional planar scans. In addition, most recent hybrid SPECT/CT systems (Schmidt et al. 2009; Kohlfuerst et al. 2009) allow a better anatomic localization of thyroid remnants or locoregional metastases (Fig. 2). In patients with large thyroid remnants, post-ablation WBS may be dominated by high thyroidal uptake which may conceals possible pathologic lesions taking up radioiodine in the neck, mediastinum or upper thorax. In such cases, diagnostic 131I WBS performed 3–6 months after ablation may be preferred for definitive staging.
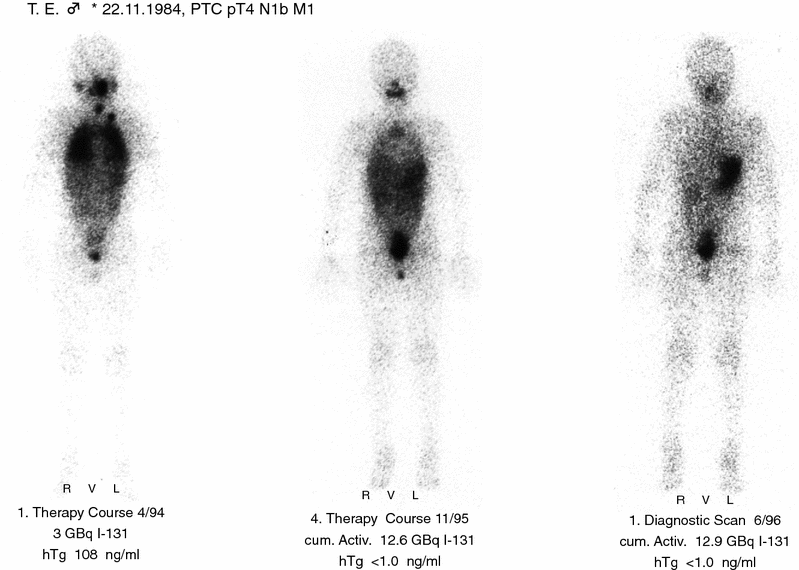
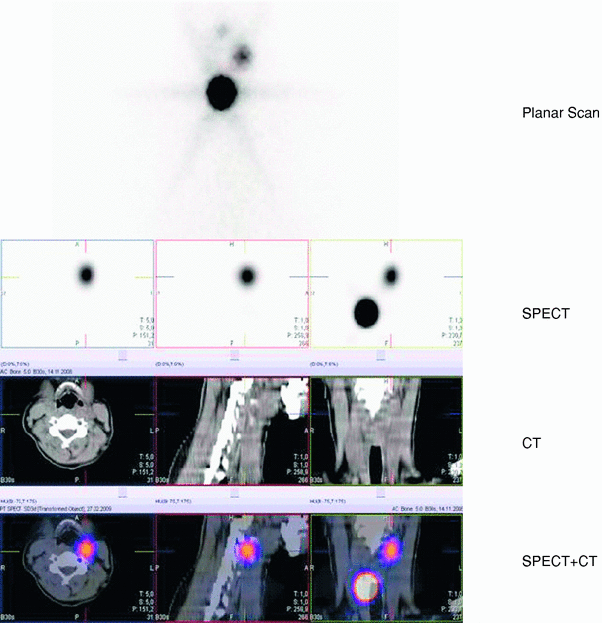

Fig. 1
Post-therapeutic 131I whole-body scans in the course of successful treatment of a young patient with papillary thyroid cancer and lung metastases

Fig. 2
Single-photon-emission computed tomography/computed tomography (SPECT/CT) scans of the neck with precise delineation of a thyroid remnant and a lymph node metastasis with intense uptake of radioiodine
7 Results Results
7.1 Results of I-131 Treatment
In nearly 70 years of experience many studies, especially those with long-term follow-up, have shown that radioiodine ablation or therapy leads to considerable clinical benefits in the sense of a reduced recurrence and death rate in DTC patients. We will highlight some of the more important studies here.
Mazzaferri and Jhiang (1994) showed that in long-term follow-up the recurrence rate was reduced from about 40 % in patients without radioiodine treatment to 10 %, whereas the death rate was reduced from 9 to 0 %. Similarly in a study by Hay et al. (2002) the long-term recurrence rate was reduced from about 25 % to about 10 % whereas thyroid cancer mortality was decreased by half. Handkiewicz-Junak et al. (2007) were able to show a reduction in long-term recurrence rates from >60 % without to about 5 % with radioiodine therapy; in a meta-analysis of literature Jarzab et al. (2005) were able to determine a relative risk for recurrence of 5.8 for those who did not receive radioiodine treatment compared to patients who did.
A large meta-analysis of the literature by Sawka et al. (2004) definitively showed that studies on the efficacy of I-131 treatment in thyroid cancer patients require a long follow-up. As can be seen in Table 3, it is only after at least 10 years of follow-up that any benefit of I-131 treatment can be proven with statistical significance. This is important to remember, as most studies in DTC do not achieve such a long follow-up. In this 2004 review by Sawka, it could not yet definitively be proven that I-131 treatment was actually beneficial to all patients.
In a further, updated meta-analysis published in 2008, Sawka et al. (2008b) tackled another difficult problem. Due to the very good prognosis of DTC patients, it is almost impossible to attain a sufficiently long-term follow-up for analysis of tumor specific mortality. Especially in low-risk patients, this would require a time-span of observation that far exceeds the length of the average physician’s career. Therefore, Sawka et al. used a surrogate measure of prognosis in order to analyze the efficacy of I-131 ablation in patients: they performed a pooled analysis of several studies reporting the later subsequent incidence of distant metastases between patients who did receive I-131 therapy and those who did not. In a pooled analysis of 2,263 patients (see Table 4) they demonstrated a small but statistically significant reduction in the risk for developing distant metastases of −2 % (95 % confidence interval: −1 to −4 %; P = 0.0005) (Sawka et al. 2008b). This significant improvement in a substitute measure can be interpreted as an improvement of the prognosis of patients after I-131 ablation.
Table 4
Efficacy of ablative radio-iodine treatment in relation to the development of metastases during long-term follow-up (adapted from Sawka et al. (2008b))
Institution | Patients (n) | Histology | Follow-up (years) | Effectiveness of I-131 ablation | P |
|---|---|---|---|---|---|
Ohio state | 1,510 | Papillary, follicular | 16.6 | With I-131: hazard ratio 0.6 (0.5–0.8) | 0.002 |
Hong Kong | 135 | Follicular | 10.8 | No significant effects | |
Hong Kong | 444 | Papillary | 9.2 | With I-131: relative risk 0.2 (0.07–0.64) | 0.006 |
Pooled | 2,263 | Papillary, follicular | Reduction of risk: −2 % (−1 to −4 %) | 0.0005 |
7.2 Prognostic Implications of the Results of I-131 Treatment
Radioiodine therapy is therefore clearly able to alter prognosis in DTC patients for the better. In fact, the response to radioiodine ablation may even be a more powerful indicator of prognosis than the initial clinicopathological stage. Verburg et al. (2010) were able to show that after successful radioiodine ablation there were no differences anymore in both recurrence and death rates between patients who were classified as low risk or high risk according the the ETA classification. Another study by Verburg et al. (2005) showed that there was a dramatic difference in both disease-free survival (87 % in case of successful ablation versus 49 % if ablation was unsuccessful) and thyroid cancer-related mortality (7 % versus 22 %) between patients in whom radioiodine ablation was successful and those in whom the treatment was unsuccessful. These findings solidly support the concept of restaging according to treatment success which was first proposed by Tuttle et al. (2008b).
Whereas radioiodine ablation often leads to great success in patients without distant metastases, the picture is looking somewhat different in patients with distant metastases. Although some patients, especially those younger than 45 years of age at the time of diagnosis, may still achieve a stable partial or even complete remission after radioiodine therapy, in many, especially older, patients distant metastases often respond poorly to radioiodine treatment or even fail to accumulate radioiodine at all. Especially in the latter case, the role of radioiodine therapy is extremely limited and other therapy modalities will have to be considered.
8 Contraindications Contraindications
Absolute contraindications (Luster et al. 2008) are pregnancy and lactation. To minimize radiation exposure to the baby and to the mother’s breast, breast-feeding should be stopped at least 6–8 weeks before radioiodine therapy. Because of resultant increased radiation exposure in the case of lactating mammae, temporarily discarding mother’s milk is not a useful alternative.
Relative contraindications (Luster et al. 2008) for radioiodine therapy in patients with thyroid cancer include:
high-grade bone-marrow depression in cases of treatment with high activities of radioiodine,
pulmonary fibrosis and considerable reduction of pulmonary function in patients with lung metastases and high radioiodine uptake,
considerable xerostomia due to proven impairment of salivary gland function.
9 Side-Effects Side-Effects
Depending on the 131I activities administered, local neck pain, sialadenitis and gastritis are frequent, non-permanent early side-effects of radioiodine treatment (Table 5). The most frequent permanent side-effect is xerostomia due to chronic sialadenitis with loss of taste and increased risk of caries sometimes accompanied by impairment of lacrimal gland function. Patients with xerostomia should be motivated to increase individual measures for prophylaxis of caries (Alexander et al. 1998).
Table 5
Side-effects of 131I therapy and potential preventive measures
Early, non-permanent side-effects | Preventive measures |
|---|---|
Swelling of thyroid remnants | Ice pads, antiphlogistics |
Sialadenitis | Induction of salivary flow with sour candy, etc |
Gastritis | Proton pump inhibitors, antiemetics |
Thrombo-, Leukopenia | Minimum interval between radioiodine therapies 6 months, blood cell count monitoring |
Swelling of metastasis | Glucocorticoids, short-term TSH stimulation |
Late, chronic side-effects | Preventive measures |
|---|---|
Xerostomia, caries, sicca syndrome | Oral hygiene and dietary prophylaxis against caries |
Bone marrow depression | Stem cell transplantation (rarely necessary) |
Secondary malignancies | Age-related early detection measures |
Pulmonary fibrosis | Minimum interval between radioiodine therapies 6 months, glucocorticoids |
Hypo-, Azoospermia | Cryopreservation of sperm |
Among other late side-effects of radioiodine treatment in thyroid cancer patients, secondary leukemia and solid cancers must be taken into account. A meta-analysis in 6,841 patients from Sweden, France and Italy showed a small, dose-dependent increase in the incidence of secondary solid tumors (of the bone, soft tissues, colon, rectum, or salivary glands) and leukemia (Rubino et al. 2003) after high cumulative therapeutic activities (>22 GBq) of 131I. In contrast, a recent American cohort study from the Surveillance, Epidemiology and End Results Registry, that included 10,000 patients given 131I for thyroid cancer and 19,000 control cases, failed to find any increased risk for secondary tumor after radioiodine treatment (Bhattacharyya and Chien 2006). Nevertheless, patients receiving radioiodine therapy for thyroid cancer, especially repeated courses for advanced disease, should be encouraged to follow age-related measures for early diagnosis of different tumors (Alexander et al. 1998).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








