Fig. 1
Frequency distribution of mean absorbed dose to the spleen in the 53 patients in the study group
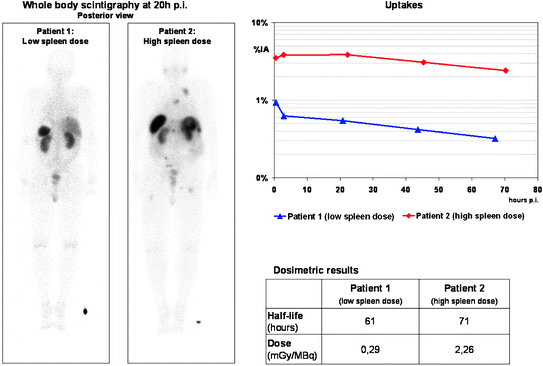
Fig. 2
177Lu posttherapy whole-body scans 20 h p.i. along with corresponding time-activity curves in two patients with high and low mean absorbed dose to the spleen
The analysis of hematological parameters revealed no significant difference (p > 0.05) in posttherapeutic changes of blood cell counts (RBC, WBC, or platelets) between the study group (Fig. 3a) and the control group (Fig. 3b). Mild hematological toxicity (erythrocytopenia and thrombocytopenia grade I) was observed in 7/53 (13.2%) of patients with intact spleen. Erythrocytopenia grade I was also observed in 1/11 (9.1%) of patients without spleen after PRRNT. In addition, there was no significant correlation between the incidence of hematological toxicity and the dose to the spleen (r 2 = 0.029; p = 0.5).
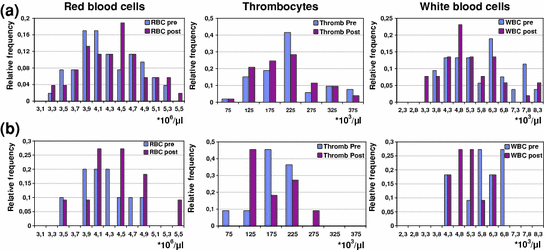

Fig. 3
Comparison between pre- and post-PRRNT blood cell counts in the study group (a) and control group (b)
4 Conclusion
PRRNT with 177Lu-labeled peptides is safe and has minor adverse hematological effects, mostly mild erythrocytopenia. However, this is most likely due to the radiation dose delivered to whole body (including bone marrow). This study demonstrates for the first time that hematological toxicity after PRRNT is not related to the radiation dose to spleen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree