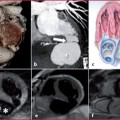
Fig. 7.1
Thoracic (a) and ascending aorta (b) anatomy
Table 7.1
Thoracic aorta
Definition | Reference calibers | Males (diameter, mm) | Females (diameter, mm) | Collateral | |
|---|---|---|---|---|---|
Ascending aorta • Annulus • Sinuses of Valsalva • Sino-tubular junction • Tubular portion of ascending aorta | Tract between the aortic valve and the origin of the first epiaortic vessel (usually, the anonymous trunk) | Sinuses of Valsalva Tubular portion | 29-35 28-36 | 25-32 26-33 | Right coronary artery Left coronary artery |
Aortic arch • Isthmus | Tract between the first epiaortic vessel and ligamentum arteriosum (the proximal and the distal portion are delimited by the origin of left subclavian artery) | Proximal arch Isthmus Distal arch | 26-34 21–30 23-31 | 24-32 20-27 2127 | Anonymous trunk Left common carotid Left subclavian |
Descending aorta | Tract between the ligamentum arteriosum and the diaphragmatic hiatus | Diaphragmatic hiatus | 22-28 | 22-28 | Bronchial Pericardial and mediastinal Oesophageal Intercostal Superior phrenic |
The anatomical variants of the thoracic aorta most commonly involve the aortic arch and mainly consist of changes in the morphology and course of the aorta (afterward discussed in malformations) and anomalies at the origin of the branches of the aortic arch.
Commonly found in healthy adult populations (25%) as an incidental finding, mostly without clinical relevance.
About 80% of these cases are at the origin of the left common carotid artery from the anonymous trunk (bovine arch).
Other less common forms are: distinct origin of the four branches from the arch, the origin of an isolated vertebral artery or the retro-esophageal course of the subclavian artery (lusory artery).
Table 7.2 shows the recommended CT acquisition protocols for different types of scanners.
Table 7.2
CTA Protocols
CM | 4 MDCT | 16 MDCT | 64 MDCT | 128 MDCT | Dual source |
|---|---|---|---|---|---|
kVp mAs | 120 120-180 | 120 120-180 | 120 120–180 (Dose modulation) | 120 120–180 (Dose modulation) | 120-120 120–180 (Dose modulation) |
Collimation Slice thickness (mm) Recon increment (mm) Reconstruction dataset Feasible ECG-gating | 4 x 2.5 mm 3 1.5-2 | 16 x 1.2 mm 1 and 3 0.5-1 | 64 x 0.6 mm 1 and 3 0.5-1 | 128 x 0.6 mm 0.5-1 and 3 0.4 | 32-64 x 0.6 mm x 2 0.5-1 and 3 0.4 |
Axial: 1 mm with medium-smooth convolution filters (threedimensional evaluation of vessels) Axial and coronal: 3 mm with smooth convolution filters (reading mediastinum and vessels) Axial: 1.5 mm with sharp convolution filters (evaluation of lung parenchyma) | |||||
No | Retrospective | Prospective retrospective | Prospective retrospective | Prospective retrospective | |
7.2 7.2 CTA Technique
Patient Preparation
Supine position with arms raised above the head.
Peripheral venous access (at least 20G cannula): possibly right upper limb to avoid artifacts during the passage of the contrast medium (cm) through the left brachiocephalic vein. Inspiratory apnea.
Image Acquisition Protocol
Scan volume: from the base of the neck to the diaphragm. If necessary, to extend the study to the neck and skull, such as in the case of suspected involvement of the upper vessels, or caudally to the abdomen and pelvis in order to properly identify injuries of blood vessels or organs in trauma patients, the continuation of an abdominal dissection or to assess the adequacy of accessing the iliac-femoral artery before an endoprosthetic placement.
Dose reduction: Use the automatic dose modulation systems (available in all of the most recent types of CT equipment) or shorten the scan time by increasing the pitch (especially with a scanner with a small number of layers), but at the price of losing image quality. The pitch should be kept between 1.3 and 1.8, or between 1.0 and 1.5 in the case of ECG-gated acquisitions.
Cardio-Synchronization
When: If you need to accurately visualize the anatomy of the ascending aorta, if you want to avoid artifacts from aortic pulsation in the case of tachycardia, if you also want to perform coronary arteries angiography.
How: the most suitable ECG-gating technique depends on the type of scanner, the heart rate and regularity of the rhythm and the need to reduce the radiation dose. Generally, we recommend:
prospective technique, if the heart rate is regular and <65 bpm;
retrospective technique, possibly with pulsed ECG-gated dose modulation, if the heart rate is irregular or > 65 bpm, or in cases of valvular evaluation, ascending aorta dissection, post-surgery complications of the ascending aorta, or a kinetic study of the heart.
Approximate estimated dose:
Full-dose ECG-gated retrospective technique : 14–20 mSv;
Pulsed ECG-gated retrospective technique: 6–12 mSv;
ECG-gated prospective technique: 1–3 mSv.
Type of contrast medium: preferably use a high-concentration contrast medium (370 and 400 mg of iodine / mL), especially in patients with poor venous access.
Flow: although in order to obtain optimal images high flows of contrast medium administration are recommended (1.6-2.0 g of iodine / s is obtained, for example, with a flow of 4.5 – 5.0 mL / s of contrast medium at a concentration of 400 mg of iodine / mL), an acceptable diagnostic quality for the study of the thoracic aorta is achievable even with poor venous access, and a flow of 1.0 g of iodine / s (which corresponds to a flow around 3.0 mL / s with a contrast medium concentration of 350 mg of iodine / mL).
Volume of contrast medium: This varies on average from 70 mL to 110 mL, and it has to be adapted to the rate of administration and to the scan duration (the latter is proportional to the scanning speed of the machine and to the anatomical volume to be covered), in order to ensure constant and adequate opacification for the entire scan.
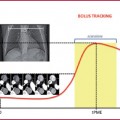
Timing of the scan: bolus tracking or test bolus techniques are always preferred, placing the region of interest (ROI) within the ascending aorta.
Particular attention should be paid to cases of dissection, intramural hematoma or aneurysm, in which the ROI could be accidentally placed in the false lumen, in the hematoma or thrombosed portion. In these cases the manual start of scanning can overcome the interference.
Bolus of saline: in order to avoid artifacts related to the presence of the contrast medium in mediastinal venous trunks, the venous access at the right arm and the addition of a normal saline bolus after the contrast medium should be preferred.
Baseline or pre-contrast scan: recommended in emergency situations, to follow-up an endoprothesis, or in the case of suspected vessel wall pathology (intramural hematoma, aortitis).
Arterial phase: this is always necessary for the study of the aorta (about 15–20 s after injection).
Late venous phase: delay of 90 s after injection. Useful in the control of aortic endoprosthesis or suspicion of extraluminal blood spreading (biphasic acquisition).
Administration protocols are suggested in Table 7.3
Table 7.3
Contrast media administration
CM | 4 MDCT | 16 MDCT | 64 MDCT | 128 MDCT | Dual source |
|---|---|---|---|---|---|
Iodine CM concentration (mgl/mL) | 350-400 | 350-400 | 350-400 | 350-400 | 350-400 |
CM volume (mL) | >110 | >80 | >80 | 70 | 70 |
Saline flush volume (mL) | 20-30 | 20-30 | 20-30 | 40 | 40 |
Flow speed (mL/s) | 3.0-4.0 | 4.0 | 4.0-5.0 | 4.0-5.0 | 4.0-5.0 |
Bolus tracking (ROI positioning and thresold value) | Ascending aorta 100–150 HU | Ascending aorta 100–150 HU | Ascending aorta 100–150 HU | Ascending aorta 100–150 HU | Ascending aorta 100–150 HU |
7.3 7.3 MRA Technique
Several MR techniques make it possible to study the aorta:
Techniques without contrast media:
Turbo spin echo sequences (TSE) 2D ECG-gated black blood Tl-weighted;
Phase contrast sequences (PC);
2D or 3D steady-state sequences, or balanced true-FISP.
Techniques with contrast media:
3D gradient-echo (GRE) Tl-weighted sequences;
2D or 3D time-resolved sequences.
The technique that is most widely accepted and applied in many clinical scenarios is MR angiography (or MRA) with Tl-weighted GRE sequences.
When evaluating the ascending aorta, aortic valve or the bulb, the examination should be integrated with ECG-gated Steady-State Free Precession Sequences (SSFP).
Non-contrast-enhanced techniques are useful when the contrast medium is contraindicated (pregnancy, renal disease) or when the subject is closely monitored.
In certain clinical situations it is useful to integrate the protocol with black blood sequences (study of wall vessel) or phase contrast sequences (hemodynamic alterations, such as coarctation or dissection).
All the sequences described, except for the breathing-triggered 3D-SSFP sequence, are acquired while the breath is held, and therefore good patient compliance is needed.
The average duration of MRA of the thoracic aorta ranges usually from 10 to 20 min, but it may extend up to 20–30 min if integrated with ECG-gated sequences.
Patient Preparation
Supine position.
Electrodes for ECG-gating.
Peripheral venous access (20–22 G cannula): preferably in the upper right limb.
Multi-channel surface coils for the chest: add a dedicated coil for the neck if you need to study the supra-aortic vessels and an abdomen coil if necessary to study the abdominal aorta and iliac vessels.
Image Acquisition Black-Blood 2D ECG-Gated Turbo Spin Echo (TSE) T1- and T2-Weighted Sequences
Conventional Tl – and T2-weighted TSE sequences, thanks to the capability of selectively suppressing the signal of circulating blood, are used in the assessment of the aortic wall (intraluminal thrombotic appositions, wall alterations such as an intramural hematoma or dissection, intimal flap, bleeding inside an atheroma, diffuse wall thickening, wall edema or peri-adventitial inflammatory phenomena).
The scan plans have to be perpendicular to the longitudinal axis of the vessel (the effect of black blood is maximized) for the study of the wall or parallel to the course of the vessel for the detection of any wall thickening.
You can also associate the selective suppression of fat signals to maximize the contrast between the aortic wall and the surrounding structures (Fig. 7.2).
The parameters are shown in Table 7.4.
ECG-Gated Phase Contrast Sequence
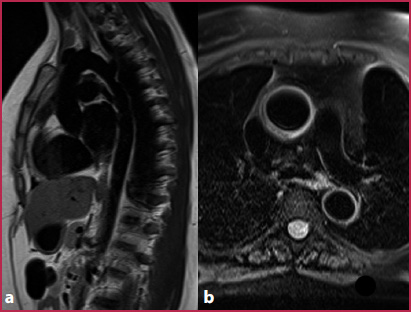
Fig. 7.2
Black blood sequences. a TSE T1-weighted oblique sagittal plane passing through the aorta. b Fat-saturated T2-weighted axial image
Table 7.4
Black blood TSE T1-weighted sequences: technical parameters
TR (ms) | 250-500 |
TE (ms) | Mininum possible |
Slice thickness (mm) | 5 |
Number of slices | 10-12 |
Acquisition time (min) | 4-7 |
ECG-gated | Yes |
The use of angiographic sequences based on the phase-contrast principle are not currently used because of the many artifacts that exist (flow void) and poor image quality.
The ECG-gated 2D PC sequences are particularly useful in qualitatively and quantitatively evaluating the hemodynamics of blood flow in specific clinical situations.
However, the complexity of their evaluation, the long acquisition times and the need for dedicated software for data processing limit their use to specialized centers, or otherwise focusing on specific clinical questions.
The optimal use of these sequences requires the following:
Preliminary assessment of the cardiovascular anatomy using morphological sequences with or without contrast;
Align plans for acquisition perpendicular to the longitudinal axis of the vessel (through-plane, qualitative and quantitative) or parallel to the vessel (in-plane, only qualitative information);
Velocity encoding factor (VENC) depending on the vessel studied and the flow velocity to be evaluated (stenosis: faster flow). Tables 7.5 and 7.6 show the technical parameters and the VENC reference.
Table 7.5
2D-ECG-gated phase contrast sequence: technical parameters
TR (ms) | Depending on the RR interval duration |
TE (ms) | 6 |
Flip angle | 20° |
Slice thickness (mm) | 6 |
Matrix | 192 x 256 |
Acquisition time (sec) | 15-25 (depending on the heart rate) |
ECG-gated | Yes |
Table 7.6
2D-ECG-gated phase contrast sequence: reference flows and proposed VENC
Reference flows | Proposed VENC | |
Aorta | <160 (cm/s) | 150-200 (cm/s) |
Stenosis of aorta or aortic valve | <400 (cm/s) | >200 (cm/s) (to assess using PC Scout or proceeding by trials) |
True FISP Sequences ECG-Gated (Balanced Steady-State Free Precession or bssFP)
These are useful in patients in whom the use of contrast media is contraindicated or not feasible (pregnancy, chronic renal failure, inability to obtain adequate venous access).
Thanks to an adequate signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) also in the ECG-gated sequences, the technique is preferable to GRE for the ascending aorta and aortic root (reduction of blurring artifact).
The single-shot images (2D) are fast and useful for quick assessment of the anatomy, even in patients unable to achieve prolonged apnea (Fig. 7.3). The technical parameters are reported in Table 7.7.
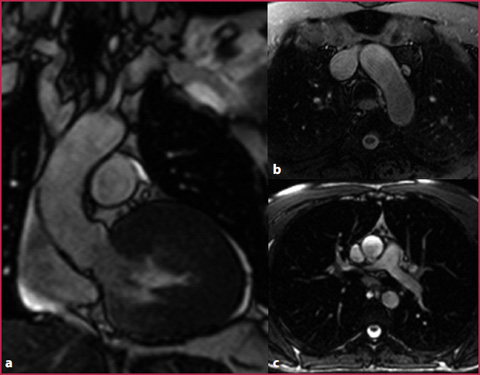
Fig. 7.3
bSSFP sequences. a Oblique coronal plane passing through the aortic root. b Axial plane passing through the arch. c Axial plane passing through the ascending aorta. This sequence can be performed with fat tissue signal suppression
Table 7.7
bSSFP sequences: technical parameters
TR (ms) | 2.3 |
TE (ms) | 1.0 |
Flip angle | 90° |
Slice thickness (mm) | 3 (interpolation of 1.5 mm) |
Matrix | 256 x 256 |
Acquisition time (min) | 4-10 (depending on the heart rate) |
ECG-gated | Yes ± respiratory navigator |
The 3D SSFP technique, already developed for the study of congenital heart disease and coronary artery angiography, makes it possible to synchronize the acquisition with both heart rate and respiratory movements (respiratory triggering), enabling high resolution free-breathing 3D acquisition, but at the cost of a prolongation of the scanning time (4–10 min).
Cine-bSSFP (2D) sequences allow a dynamic display of valvular and vascular structures with characteristic signal abnormalities in cases of stenosis (flow void), insufficiency (regurgitation counter) or turbulent flow.
3D GRE T1-Weighted Sequence
These are most often used because of their speed and the anatomical detail provided (Fig. 7.4). They allow high spatial resolution (isotropic or semi-isotropic voxel, submillimeter resolution and minimum matrix of 384 x 384) (Table 7.8) with excellent temporal resolution (acquisition time between 10 s and 20 s, obtainable in a breath hold).
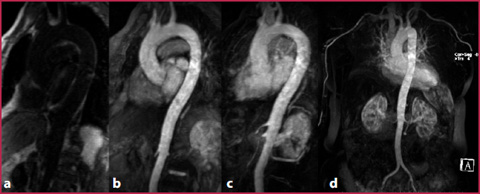
Fig. 7.4
GRE-T1 weighted sequences. Oblique sagittal plane performed before contrast media administration (a), on first-pass arterial phase (b). Post-processing makes it possible to obtain subtracted images (c) and MIP (d)
Table 7.8
GRE sequences: technical parameters
TR (ms) | 2.8 |
TE (ms) | 1.4 |
Flip angle | 25° |
Slice thickness (mm) | 0.8 |
Matrix | 384 x 384 |
Acquisition time (s) | 15-20 |
ECG-gated | No |
Furthermore, the subtraction from the contrast-enhanced images of the pre-contrast mask removes the extravascular tissues, improving the contrast resolution of the images.
ECG-gating is possible, at the price of an increase in acquisition time (30–40 s) and a lower sharpness of the image.
Scan Protocol
Localizer acquisition on the three planes of space;
Mask acquisition (3D GRE) preferably on the oblique sagittal plane parallel to the aortic arch course (in the axial images, the center of the package must pass through the ascending and descending aorta);
For precise timing of contrast medium, use the Fluoro-MR technique by monitoring the passage of contrast medium through the right chambers and starting the contrast-enhanced sequence as soon as the bolus reaches the left ventricle, or using a preset delay of 20s;
Acquisition of Contrast-Enhanced Sequence (3D GRE identical to the mask);
Subtraction of the Contrast-Enhanced scan from the mask (it nullifies the signal from extravascular stationary tissue, in particular from fat that is hyperintense on T1, and minimizes wrap around artifacts);
You should always perform a second acquisition, both for more diagnostic images useful in the case of artifacts in the first acquisition, and to evaluate venous vascular anomalies.
Time-Resolved Sequence
This enables the visualization of bolus transition by serial acquisitions after contrast medium administration, at the price of a reduced spatial resolution. These sequences are useful for evaluating anatomical anomalies (for example the coarctation), dissections and in the suspicion of cardiovascular shunts (Fig. 7.5, Table 7.9).
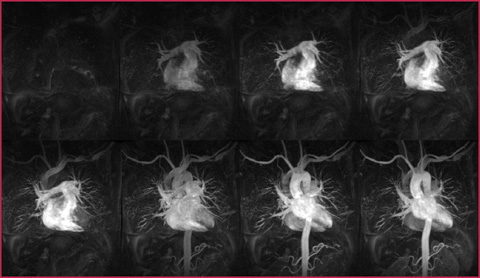
Fig. 7.5
Serial acquisition of scan volumes including all mediastinal vascular structures. To each acquisition the former is subtracted to allow the visualization of the aorta without the overlapping of surrounding venous and pulmonary structures
Table 7.9
3D Time-resolved sequences: contrast media administration
CM concentration | 0.5 M |
CM volume | 10 mL (0.1-0.2 mL/kg) |
Saline flush volume | 20 mL |
Flow volume | 1.5-2 mL/s |
Localizer planes on the three dimensions;
Mask acquisition;
Images are acquired simultaneously with contrast medium administration (free-breathing), visualizing the first pass;
Optionally you can perform the subtraction of the contrast-enhanced sequence from the mask and get a Maximum Intensity Projection (MIP) reconstruction for each volume acquired.
7.4 7.4 Congenital Aortic Malformations
Arch Anomalies
Embryonic origin: up to the 4th-8th week of gestation, the arterial vascular anatomy consists of a ventral aortic trunk, which splits into two arches and rejoins into a single dorsal trunk. The selective spontaneous obliteration of some portions of this vascular ring and subsequent enlargement of the remaining structures determine the anatomical conformation in the adult (Fig. 7.6).
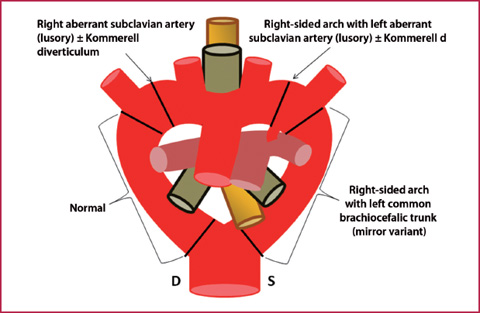
Fig. 7.6
Embryonic arterial circulation (anterior view). The scheme shows how selective obliteration of different tracts of the vascular ring (black lines) leads to corresponding different vascular abnormalities
Normal anatomy: normally the right arch regresses in the portion between the origin of the ipsilateral subclavian artery and the rejoining with the left arch.
Congenital Anomalies: Congenital arch anomalies originate from an alteration of this process and can result in the persistence of a so-called vascular ring. These conditions may be completely asymptomatic or clinically manifest due to compression of the trachea and/or esophagus (dyspnea, respiratory infections, dysphagia). Moreover, they are often associated with other congenital cardiovascular abnormalities and predispose to serious complications such as rupture or dissection.
Imaging and Reporting
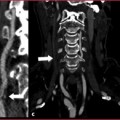
Double aortic arch: this is the most common complete vascular ring, with all portions of embryonic aorta preserved, even though one of the two arches can be dominant and hypertrophic compared to the contralateral (Fig. 7.7).
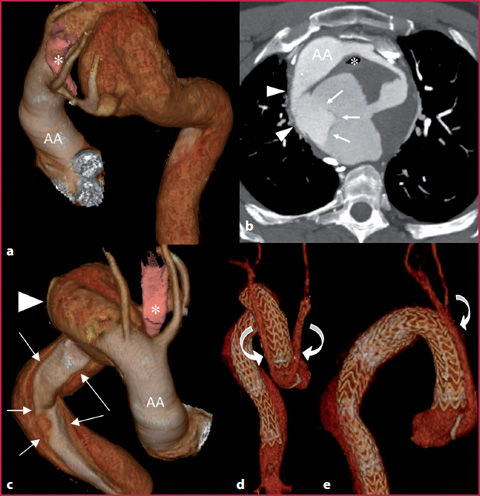
Fig. 7.7
a-c CTA of a double aortic arch, with right arch dominance (white arrowheads) and a dissection at the confluence of arches. Complete vascular ring causes tracheo-esophageal compression (asterisk). The dissection (white arrows) runs from the arches to the abdominal aorta, while the ascending tract (AA) is normal. d-e After supra-aortic vessels debranching (curved arrows) and ascending aorta bypassing, three endoprosthesis are placed to cover the whole thoracic aorta and to stabilize the dissection
Right-sided aortic arch: it is generated from the obliteration of the left arch, and may have a left anonymous trunk (mirror image branching) or left retroesophageal subclavian artery (lusory), depending on which tract is obliterated.
Diverticulum of Kommerell: often associated with a right sided arch, this is an aneurysmal dilation of the aberrant subclavian artery at the origin, determined by atresia of the portion of the contralateral arch between the origin of the carotid artery and the subclavian artery.
Treatment Patent Ductus Arteriosus
Surgery, Only in Case of Symptoms or Complications
Get Clinical Tree app for offline access
• The ductus arteriosus or Botallo’s duct is a small arterial duct connecting the pulmonary artery and the aorta. It allows blood circulation during fetal life and normally becomes spontaneously obliterated a few hours or days after birth, as it is no longer necessary.
Sometimes the spontaneous closure of the ductus does not happen, the ductus remains patent and a left-to-right shunt of variable significance may persist.
The finding is often incidental. It assumes clinical relevance only when it forms a complete or incomplete vascular ring, or if it leads to a hemodynamically significant shunt. (Fig. 7.8)
Congenital narrowing of tubular aorta most often occurs at the aortic isthmus; if slight it may remain unrecognized until adulthood and it is often associated with a bicuspid aortic valve, ventricular septal defect or other congenital anomalies.
The clinical scenario is variable and depends on the development of collateral circulation through the internal mammary artery, intercostal arteries, thyreocervical or costocervical trunks, to overcome the hemodynamic obstacle, reverting the blood flow to the descending aorta.

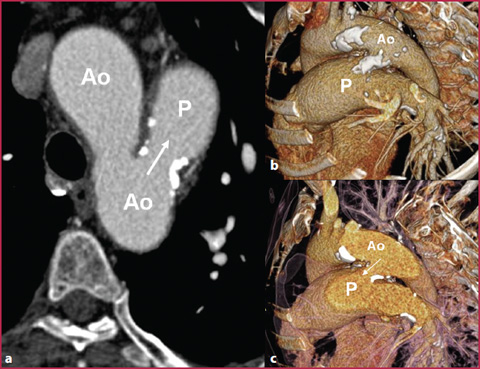
Fig. 7.8
CTA axial (a) and VR (b, c) reconstructions of a 53-y-old patient with a huge patent ductus arteriosus (white arrows) between the aortic arch (Ao) and the pulmonary artery (P




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree