Chapter 30 Thoracic Metastatic Disease
Introduction
Metastatic disease is the most common chest malignancy and the chest acquires more metastases than any system.1 In autopsy series, pulmonary metastases are present in 20% to 54% of patients with a primary malignancy.2,3 The most common extrathoracic malignancies to metastasize to the chest include breast cancer, gastrointestinal (GI) malignancies (colon, pancreatic, and gastric cancer), melanoma, head and neck tumors, and renal cell cancer.1 Rarely, metastatic disease to the thorax can be the initial presentation of a malignancy. Tumor spread to the chest can occur via hematogenous, lymphatic, or endobronchial routes. Endobronchial spread most commonly occurs in primary lung adenocarcinoma and from tumors of the upper airway and paranasal sinuses. Chest radiographs and computed tomography (CT) are the main modalities used to assess thoracic metastatic disease. Other modalities currently serve complementary roles and include magnetic resonance imaging (MRI), ultrasound (US) and positron emission tomography (PET)/CT. Technique and typical and atypical CT imaging manifestations of pulmonary metastases, pleural metastases, and cardiac metastases are addressed.
Technique
Imaging is indispensable in the evaluation of patients with a primary tumor. In these patients, the principal functions of imaging are to stage patients and to detect or exclude the presence of metastatic disease. Achieving this goal with the appropriate imaging technique is essential. Chest radiographs, posteroanterior and lateral views, generally serve as the initial evaluation of patients with possible metastatic disease. In patients whose radiographs demonstrate obvious signs of metastatic disease, additional imaging modalities may not be necessary and chest radiographic follow-up may be all that is needed.4 However, chest radiographs lack sensitivity and CT scanning has been shown to be superior to chest radiographs in the detection of pulmonary nodules. CT scanning is now accepted as the state-of-the-art modality in the search for metastatic disease and in characterizing lesions in the thorax.5,6 The evolution of CT as the standard began with improvements in CT technology that include the ability to obtain thinner collimation and development of spiral and subsequently multidetector row computed tomography (MDCT) scanners. Thinner collimation brought on the development of the science of high-resolution computed tomography (HRCT) to assess diffuse lung disease, improved anatomic assessment and characterization of small lesions. Current MDCT scanners obtain a continuous volume acquisition and, thus, decrease misregistration artifacts and improve nodule detection by eliminating interslice gaps. This aids assessment of diffuse lung diseases, increases our sensitivity for small lung nodules, and improves multiplanar image reconstructions. In addition to the increased sensitivity provided by CT scanning, other benefits include its ability to quantify disease in the case of possible metastectomy, assess response to therapy on sequential studies, and provide a detailed roadmap to guide biopsy.
Our present CT chest protocol for assessing patients with possible metastatic disease include MDCT scan parameters that allow image review in both standard and lung algorithms at a 2.5-mm image thickness. The CT parameters also permit 1.25-mm-thick image reconstructions to be performed retrospectively, as needed. Images (2.5-mm image thickness) are also reconstructed in both the coronal and the sagittal planes. Images are reviewed at workstations that have cine mode capabilities.
1. Contrast enhancement permits a more exact delineation of the mediastinum and hilar regions and is also useful to detect subtle pleural metastatic disease.
2. Patients with primary malignancy are at increased risk for developing pulmonary emboli. In retrospective reviews of CT scans of oncologic patients, between 3.3% and 4.0% of patients had incidentally discovered pulmonary emboli with higher risks associated with inpatients, advanced disease, gynecologic malignancy, and melanoma.7,8
3. Patients undergoing chemotherapy often have indwelling catheters that may lead to thrombotic occlusion of vessels and visualization of collateral vessels.
The advances in MDCT, changes in image review from hard-copy film to workstations, and the use of the cine mode have dramatically improved our sensitivity to small solid lesions.9 In addition, sensitivity in detection of nonsolid and semisolid lesions has increased. It is widely accepted that CT scanning is a sensitive but nonspecific modality in assessing metastatic disease. False-positive nodules are often caused by intraparenchymal lymph nodes and noncalcified granulomas. Image processing can also improve image interpretation. It has been shown that the addition of maximum intensity projection images, by demonstrating vascular structures as clearly tubular branching structures and enhanced anatomic orientation, increases reader sensitivity to small lung nodules.10
Other modalities such as US, MRI, and PET serve complementary roles in evaluating metastatic disease of the thorax. Electrocardiogram (ECG)-gated cardiac magnetic resonance imaging (CMRI) is useful in evaluating the heart and surrounding cardiac structures for questionable findings on staging CT and equivocal findings on echocardiography that may suggest metastatic disease. CMRI is a cardiac-gated study that eliminates cardiac motion and has excellent temporal resolution and excellent soft tissue contrast. In a similar vein, ECG-gated CT has emerged as an alternative to CMRI in the evaluation of cardiac tumors in patients who are unable to undergo CMRI, for example, those patients who have an implanted ferromagnetic device or discomfort lying on the MRI table for prolonged periods of time.11
Patterns of Metastatic Disease
Simple Nodules
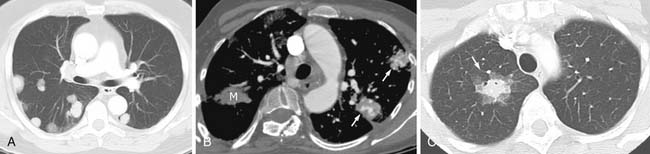
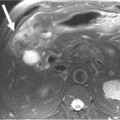
Most metastases to the chest are spread hematogenously. The characteristic appearance of these metastatic lesions include multiple bilateral peripheral spherical or ovoid sharply marginated nodules of variable sizes predominantly affecting the periphery of the lungs and the lower lobes (where the majority of the blood flow is directed)1,3,12 (Figure 30-1A). The typical sharply marginated edges of metastatic lesions help differentiate these tumors from primary lung cancer that normally show ill-defined, spiculated margins that extend into the adjacent lung parenchyma. Growth of metastatic lesion is extremely variable and volume doubling times, an increase in the diameter of a nodule by 26%, have been reported to range from 1 to 2 weeks for rapidly growing lesions such as sarcomas, melanomas, and germ cell tumors to months for thyroid malignancy.13 Hematogenous spread of metastases can also simulate a miliary pattern, typical of medullary thyroid malignancy.1 Occasionally, imaging metastatic nodules with contrast-infused CT imaging demonstrates areas of enhancement with dilated, tortuous tubular enhancing vessels. This feature can be seen in sarcomas, particularly alveolar soft part sarcoma or leiomyosarcoma6,14 (see Figure 30-1B).
Benign tumors rarely metastasize, but occasionally, metastases are found in the lung. Benign tumors that have been shown to metastasize to the lung include leiomyoma of the uterus, hydatidiform mole, giant cell tumor of bone, chondroblastoma, pleomorphic adenoma of the salivary gland, and meningioma. Slow growth of these solid benign nodules is typical but these are indistinguishable from other malignant metastatic nodules.6
Occasionally, metastatic nodules followed on sequential scans may not change in appearance and these nodules may, in fact, represent sterilized metastatic disease. These residual nodules are indistinguishable from viable tumor on CT scans. Biopsied materials show areas of necrosis and/or areas of fibrosis. Testicular cancer, breast cancer, and choriocarcinoma are common tumors that present in this manner.15,16 In some cases, following serum markers such as beta-human chorionic gonadotropin, alphafetoprotein, or PET scanning can be helpful in following and assessing viability. In addition, growth of metastatic nonseminomatous germ cell lesions with negative serum markers frequently represents a conversion to a benign mature teratoma.17
Nodules with a Ground Glass “Halo”
Hemorrhage around a metastatic lesion can create the appearance of a solid nodule surrounded by a ground glass rim, known as the CT halo sign. Fragility of the neovascular bed is a proposed mechanism. Metastatic tumors that can be associated with this appearance include choriocarcinoma and angiosarcoma among other lesions18,19 (see Figure 30-1C). The appearance is not pathognomonic for metastatic disease and can be seen in other lesions that bleed including infections such as invasive aspergillosis (the most common condition showing the CT halo sign in immunocompromised patients) and inflammatory processes such as Wegener’s granulomatosis. In addition, primary malignancy can simulate this appearance; examples include minimally invasive adenocarcinoma (the most common condition showing the CT halo sign in immunocompetent patients) and lymphoma.6,19
Cavitary Nodules
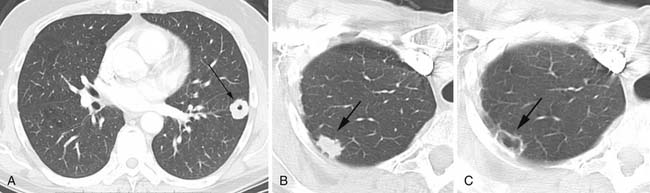
Cavitation in metastases is less frequent than in primary lung cancers. Metastatic squamous cell lesions, from any source, were thought to have the highest rate of cavitation, approximately 10%. However, a similar rate of cavitation was demonstrated in a series of metastatic adenocarcinoma.6 Typical cavitary metastases have thick irregular walls (Figure 30-2A). Less frequently, cavitary metastases can have thin walls and this feature is particularly noted in patients with metastatic adenocarcinoma or sarcoma. Chemotherapy can induce cavitation in lesions, and this is more common in sarcomatous metastatic lesions than in other cancers1,6,20 (see Figure 30-2B and C).
Calcified Nodules
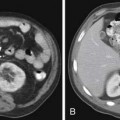
Calcification is often considered a sign of benignity, representing either a granuloma or a hamartoma; however, infrequently calcification can occur in a variety of metastatic lesions through different mechanisms. Most frequently, calcification in metastases is associated with matrix-forming primary tumors such as osteosarcoma and chondrosarcoma12,21 (Figure 30-3A). Mucoid-producing tumors can lead to calcified metastases and include tumors of the GI tract and breast cancer (see Figure 30-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree