Lawrence J. Keating • Ashley Adamovich
ABDOMINAL TRAUMA
BACKGROUND
During the past three decades, management of traumatic abdominal organ injury has evolved from a predominantly surgical approach to a strategy of nonoperative therapy in most cases. There is now broad consensus that most patients with injuries to solid abdominal viscera who are hemodynamically stable are candidates for nonoperative management (NOM), although debate persists about the specifics including when to use angiography and which patients should proceed directly to surgery. The change in philosophy has been in large part a result of rapid advances in imaging capabilities, specifically computed tomography (CT), and the increasing capability of interventional radiology to treat vascular injuries using minimally invasive endovascular techniques. During the 1980s, diagnostic peritoneal lavage (DPL) was obviated by the development and availability of CT. With concurrent expansion in the capabilities of interventional radiology, the risks of surgery began to outweigh the risks of conservative management, defined as observation with or without angiography and embolization, in many cases.
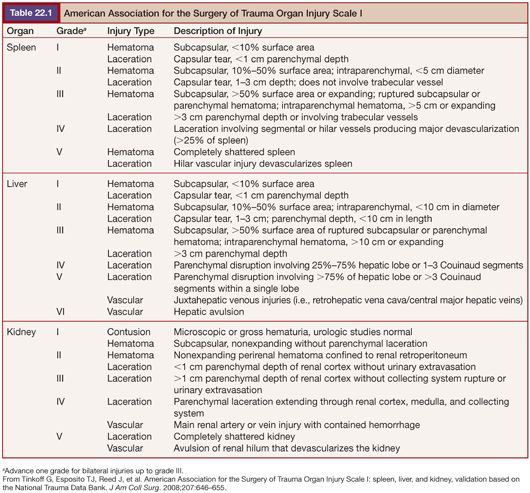
The Organ Injury Scale (OIS)—created by a committee of the American Association for the Surgery of Trauma (AAST) in 1987 and since updated and validated for the liver, kidney, and spleen using National Trauma Data Bank (NTDB)—is the most commonly used grading system for evaluation of abdominal trauma (Table 22.1).1 The current scale was revised in 1994 for the spleen and liver, partly to reflect the increasing reliance on CT for diagnosis and grading. It provides a common nomenclature for studies and outcomes research that is used almost exclusively in the trauma literature, although it is imperfect, particularly when used to assign prognostic value, which is not its fundamental objective.1,2 It is designed primarily to allow comparison of equivalent injuries managed differently.3 In a study designed to evaluate and compare injury grading scales, Barquist et al.4 found significant interreader variability among radiologists in higher grade injuries as well as a tendency to underestimate injury grades based on CT compared to operative findings. Contrast extravasation indicating continuing hemorrhage and vascular injuries such as pseudoaneurysm or arteriovenous fistula are prognostic factors and may be indications for nonoperative interventions such as angioembolization but are not specifically described in the scale. Modifications addressing these concerns including a new CT grading system for splenic injuries and substratification of the renal OIS grade IV into IVa (high risk) and IVb (low risk) have been proposed but not yet widely adopted.5,6
The OIS enables accurate outcomes comparisons among institutions with differing protocols, but urgent decisions in the trauma setting are typically made based on clinical status combined with imaging findings. Current surgical guidelines support emergency surgery for hemodynamically unstable patients (hypotension and tachycardia unresponsive to ongoing fluid and packed red blood cell resuscitation) and a trial of NOM for stable patients in settings where monitored beds and surgical teams are readily available.6–10
The new standard of conservative management has been supported and improved by the increasing availability and effectiveness of angiography with embolization. By 2005, up to 85% of traumatic injuries involving liver, spleen, and kidney were managed nonsurgically.10 Shafi et al.11 studied operative intervention and mortality rates at 152 level I and level II trauma centers and demonstrated that hospitals with higher risk-adjusted mortality rates tend to also have the highest rates of surgical intervention for abdominal trauma. Various conclusions can be drawn from this, but the lowest mortality rates will occur when we have the best understanding of which patients need operative versus conservative management. Because randomized trials are impractical in this setting, collective experience in the form of retrospective studies is our principal guide.
SPLEEN
The spleen may be the most commonly injured organ in blunt abdominal trauma.12 Aristotle considered the spleen an unnecessary organ and this was the prevailing view until recently.13 From the first splenectomy for trauma by Nicholaus Matthias in 1678 until the 1970s, removal of the injured spleen was accepted, if not standard, practice. The notion that without splenectomy most patients with splenic injury would bleed to death because the spleen cannot heal nor be easily repaired was rarely questioned.13 Interest in splenic preservation increased after the description in 1952 by King and Schumacker14 of overwhelming postsplenectomy infection (OPSI) in infants. Upadhyaya and Simpson performed the first case-control study of operative versus nonoperative management for pediatric splenic injury and demonstrated the safety of the latter approach in 1968.13 Children were found to recover from these injuries surprisingly well, and by the late 1980s, the conservative management of pediatric splenic trauma was universally accepted, a change largely predating the radiologic advances that were crucial to the broad adoption of this strategy in adults.14,15
New understanding about the spleen’s role in immunocompetence encouraged early efforts at salvage in adult trauma patients. OPSI, although rare, with a lifetime risk of less than 0.05%, carries a mortality rate of 50%.12,16 Less severe infectious complications are more common, including abscess, wound infection, and pneumonia, all of which are increased after splenectomy, compared to splenic preservation after trauma.17 The reasons for this remain incompletely understood. The spleen represents one-quarter to one-half of the lymphoid tissue in the body; is a reservoir of macrophages, which remove bacteria and red blood cells infected with parasites; and produces vital immunomodulators such as opsonins, which are needed to clear encapsulated organisms. Asplenic patients are probably more susceptible to gram-negative bacteria and fungi as well.12 Splenectomized patients should be immunized against Streptococcus pneumoniae, meningococcus, and Haemophilus influenzae type B, the encapsulated organisms for which vaccines are currently available.12,16,17
The trend toward conservative management of splenic injury coincided with the development of endovascular techniques to achieve hemostasis and support organ preservation. Although balloon occlusion and gelatin foam embolization had been previously reported,18,19 in 1981, Sclafani20 described endovascular occlusion of the proximal main splenic artery with coils, and he predicted that this would improve the outcomes of NOM. In 1991, Sclafani and his colleagues21 reported a striking 97% splenic salvage rate with routine angiography and selective proximal splenic artery occlusion in patients with splenic lacerations diagnosed with CT.
The specific indications and most appropriate candidates for NOM and adjunctive angioembolization have been a topic of debate and many retrospective studies in the intervening period; the object has been to elucidate the vital factors which contribute to the success or failure of conservative management. Failure is indicated by continued or recurrent splenic bleeding, often referred to as delayed splenic rupture. Peitzman et al.22 found that the success of NOM is directly correlated with increasing hematocrit and blood pressure and inversely correlated with OIS grade and quantity of hemoperitoneum. Advanced age has been shown to be a risk factor; Renzulli et al.23 found age older than 55 years to be the only independent risk factor for failure of NOM. The direct relationship between increasing OIS grade and failure rate of conservative therapy has been demonstrated in multiple retrospective analyses.22,24–27
Patient Selection
Most large trauma centers include splenic artery embolization (SAE) as a variable component of NOM. Much of the current literature supports angiography for hemodynamically stable patients with CT findings suggesting contrast extravasation and/or grade IV or V injuries. Several studies have demonstrated that in low-grade injuries (OIS I to III), angioembolization does not result in an improvement in outcomes, whereas in higher grade injuries, a marked improvement is seen.25–27 For example, Requarth et al.27 showed that although failure of nonoperative management (FNOM) was less than 5% in OIS grades I and II injuries with or without SAE, it rose with each OIS grade to 83.1% in grade V observation-only patients but only to 25% in patients who underwent SAE.
Although most trauma centers include angiography and embolization as an adjunct to NOM of splenic trauma in hemodynamically stable patients, there are no randomized trials. Thus, the Eastern Association for the Surgery of Trauma (EAST) assigns a level 2 recommendation to use SAE in grades IV and V injuries or whenever contrast extravasation is noted on CT.8 Bhullar et al.28 supported this recommendation in a 2013 study, pointing out that significantly higher failure rate of NOM in grades IV and V injuries may be affected by the fact that many centers do not perform angiography in cases where extravasation is not noted on CT. Although evidence of active bleeding is more common in higher grade injuries, it may be seen in lower grade (OIS I to III) injuries as well.
Technique
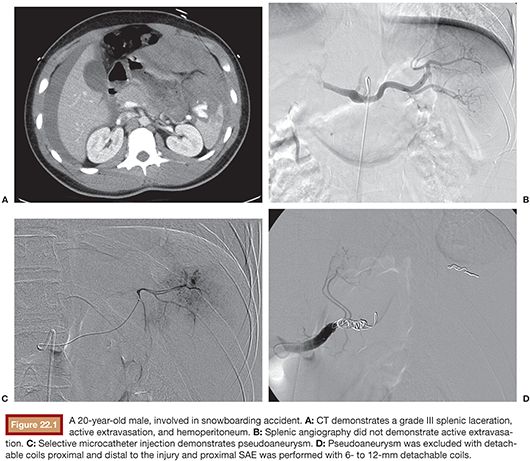
If the splenic artery is clearly identified on the admission CT, a flush aortogram may not be necessary before splenic artery selection. Typically, a Cobra (Angiodynamics, Latham, New York) or reverse curve catheter such as an Sos or Mikaelsson (Angiodynamics, Latham, New York) is used to select the celiac axis. Splenic angiography should be performed with automated injection. If angiography reveals active extravasation, then selective distal coil embolization may be performed using a microcatheter with microcoils and/or gelfoam, followed by proximal main splenic artery coil embolization (Fig. 22.1). If there is no evidence of active hemorrhage, then only proximal main SAE is performed using coils, either via the main catheter or a microcatheter.
The rationale for proximal main SAE is reduction of splenic blood pressure, facilitating hemostasis without causing infarction. The abundant arterial supply to the spleen makes this possible. Perfusion is maintained by pancreatic, omental, and short gastric arteries at relatively lower pressure, which gives splenic vascular injuries an opportunity to heal; as the collateral arteries enlarge, pressure is believed to eventually return to preembolization levels, although when this happens is unknown.29,30 Requarth and colleagues30 conducted a study demonstrating significant variability in the distal splenic arterial pressure during proximal balloon occlusion of the splenic artery. They concluded that some patients, such as those with celiac stenosis, might already have well-developed splanchnic collaterals, which would negate the impact of proximal splenic artery occlusion on parenchymal pressure. Interestingly, their results suggest that it may be reasonable to perform splenic artery balloon occlusion with pressure measurements in all splenic trauma patients before deciding whether to embolize; patients who do not demonstrate a significant decrease in splenic artery pressure during balloon occlusion may be better served with either surgery or observation.
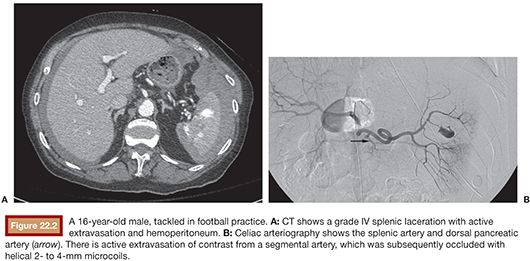
The diameter of the splenic artery should be measured and coils oversized by at least 2 mm to avoid coil migration and increased risk of splenic infarction. Appropriate sizing is difficult. Detachable coils allow the operator to retract a partially deployed coil if it appears that migration is likely. For proximal main SAE, coils should be placed distal to the dorsal pancreatic artery and proximal to the greater pancreatic artery (often called by its Latin name arteria pancreatica magna), although the ideal location is not known (Fig. 22.2). The dorsal pancreatic artery is usually the largest splenic artery branch to the pancreas and there is at least a small risk that occluding this vessel could lead to pancreatic ischemia.29 It also gives rise to distal branches that become a collateral source of splenic perfusion after occlusion of the splenic artery. However, there is variability in the anatomic origins of these pancreatic branches, and they cannot always be identified with certainty. The omental and short gastric arteries, left gastroepiploic artery, and other branches from the inferior and caudal pancreatic artery will also serve as collateral blood sources for the spleen after proximal embolization.29,30 Postembolization angiography should demonstrate occlusion of the main splenic artery with delayed splenic parenchymal perfusion via collateral flow.
Results
In a comprehensive retrospective analysis of 33 blunt splenic injury outcomes articles from 1994 to 2009 by Requarth et al.,27 patients were stratified based on type of NOM (with or without SAE) as well as splenic injury grade. They found the overall failure rate of observational management to be 17%, with much worse rates of 44% and 83% in grades IV and V injuries, respectively.27 However, SAE significantly decreased the failure rates in grades IV and V to 17% and 25%, respectively.27 Bhullar et al.28 found a 4% failure rate in patients with high-grade splenic injuries who underwent SAE, including those with contrast blush on CT, only 9% of whom ultimately required laparotomy (splenectomy or splenorrhaphy). In one of the largest single-center studies using a protocol of selective embolization in patients with CT evidence of vascular injury or active bleeding, Sabe et al.31 reported an NOM success rate of 97%. Banerjee et al.32 compared outcomes across four level I trauma centers with varying rates of embolization and found that SAE is an independent predictor of spleen salvage; centers in which it was used more had higher NOM success rates. Haan et al.33 published another large single-center study which demonstrated 90% success overall with NOM and over 80% success in grades IV and V splenic injuries. Many of the successful cases had CT scans demonstrating pseudoaneurysm or active extravasation and were treated with SAE. However, in patients with traumatic arteriovenous fistula (AVF), failure rates were high (40%) even after SAE. They concluded that AVF requires direct embolization and that proximal SAE is insufficient in these cases.33
In cases of late rebleeding after observation or SAE, it appears that many, if not most, centers favor splenectomy even though conservative management has become standard therapy for acute splenic injury. The reasons for this are unclear but likely reflect a reluctance to continue with a “failed” strategy. In a paper by Liu et al.,34 15 cases of “delayed splenic rupture” were reviewed. Twelve were treated nonoperatively with 83% success rate, and 5 of these underwent SAE with 80% success rate. These results are comparable to those of primary NOM with or without SAE and they conclude that embolization is a reasonable strategy for late rebleeding.34
The most common complication directly related to SAE is splenic infarction, of which there is a higher risk when distal embolization is performed.35,36 The clinical significance of these typically small or segmental splenic infarcts is unclear, as most ultimately resolve without further intervention.35 In a meta-analysis by Schnuringer et al.,35 no difference in the rate of major complications such as large infarct or abscess requiring splenectomy was found when comparing proximal and distal embolization techniques. Other complications are predominantly technical and rare, including arterial dissection, coil migration into the aorta, and femoral artery pseudoaneurysm.36
Protocols regarding observation, discharge, and follow-up imaging vary but typically include inpatient stay of 3 to 5 days, as recommended by Peitzman et al.22 Rebleeding, the most common cause of failure, most often occurs within 3 days of injury.37,38 Smith et al.38 demonstrated that 95% of failures would be detected within 3 days. Significantly improving this risk is unlikely because statistically, to detect 99% of failures, 30-day observation would be required.38 Most surgeons do not perform routine postdischarge imaging.9 This is supported by a study by Haan and colleagues37 examining splenic pseudoaneurysms after NOM. In their series, distal splenic embolization was only performed if free extravasation of contrast was seen at angiography. Pseudoaneurysm, AVF, and extravasation confined to the spleen were treated with proximal SAE. Patients found to have persistent or new pseudoaneurysms on follow-up CT after NOM had similar splenic salvage rates (94%) without additional therapies. Most pseudoaneurysms had resolved on follow-up imaging.37
Finally, the question of immunocompetence after splenic angioembolization has been addressed in several papers. Although our understanding of immunomodulating functions of the spleen is incomplete, authors of several studies have concluded that there is no evidence that immune function is significantly affected by SAE.17,39 Therefore, immunization is not recommended for these patients.
TIPS AND TRICKS
• When performing a proximal SAE, ideal coil deployment is between the dorsal pancreatic and great pancreatic artery (also known as arteria pancreatica magna). Given the anatomic variability and often poor visualization of these branches, a good rule of thumb is to deposit coils at the junction of the proximal and middle third of the splenic artery.
• Sizing coils for a proximal SAE can be difficult. Detachable coils or Amplatzer Vascular Plugs (St. Jude Medical, Inc., St. Paul, Minnesota) may be partially deployed and retrieved if they do not “hold,” which helps to avoid distal coil migration.
• Selective distal coil occlusion should only be performed if there is active extravasation, pseudoaneurysm, or AVF. Given the likelihood in high-grade injuries of other vascular lesions that may not be evident on angiography due to thrombus or vasospasm, this should be followed by proximal SAE.
LIVER
Hepatic arterial embolization, similar to splenic embolization, is an important adjunct in the NOM of liver trauma, although technique, rationale, and complications are different. Owing to the greater inherent difficulty of controlling hemorrhage from hepatic compared to splenic injury, angiography and embolization may play a larger role during and after surgery.40 This is because high-OIS-grade liver injuries often produce arterial bleeding, which is well controlled by transarterial embolization, as well as venous bleeding, which is not.41 Juxtahepatic venous hemorrhage often requires laparotomy, sometimes with perihepatic packing and temporary closure (“damage control”) for the most critical patients.40–42 In many modern operating rooms which are equipped with adequate fluoroscopy, embolization of deep, surgically inaccessible arterial bleeding can be accomplished immediately after laparotomy.
Identifying the patients with injuries to the retrohepatic inferior vena cava and hepatic veins therefore is vital. In a 2003 paper by Mohr et al.,43 patients with juxtahepatic venous injuries had the highest mortality rates among liver injuries. However, according to Hagiwara and colleagues,41 CT has low specificity and positive predictive value for venous injury. In their 2002 prospective study of liver trauma patients, the highest sensitivity, specificity, and positive predictive value of juxtahepatic venous injury was resuscitative requirement of greater than 2 L of fluids per hour.41 Although most would agree that these patients are not stable and should be brought to the operating room, in a 2009 paper by Misselbeck and colleagues,44 52% of patients who underwent laparotomy for hepatic injury demonstrated continued postoperative arterial bleeding requiring embolization. In the same study, patients with CT evidence of active extravasation were 20 times more likely to have positive angiograms compared to those with no evidence of active hemorrhage on CT.44 The 2012 EAST guidelines assign a level 2 recommendation to angiography with embolization in patients who are transient responders to resuscitation as an “adjunct to potential operative intervention.”7
Technique
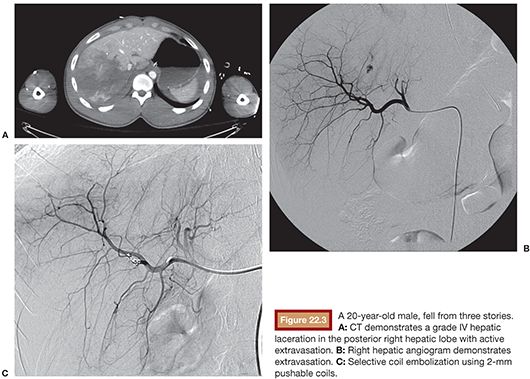
Hepatic angiography should generally begin with flush aortography due to the high incidence of variable anatomy. A 5-Fr catheter is used to select the celiac axis and angiography is performed from the common hepatic artery. Even if there is no evidence of hemorrhage, selective angiography should be performed with a microcatheter targeting areas of extravasation identified on CT. In contrast to proximal splenic artery occlusion in which decreasing blood pressure to the spleen is the primary goal, the goal in hepatic injury is to embolize distally where there is evidence of hemorrhage (Fig. 22.3). The dual arterial and portal venous blood supply to the liver likely confers some protection from ischemic complications, but proximal hepatic artery occlusion is typically unnecessary and may be detrimental, particularly in patients with preexisting liver disease or compromised portal venous blood flow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree