Organ/anatomical area
Present and/or normal?
Head
Present
Cranial bones
Midline falx
Choroid-plexus-filled ventricles
Neck
Normal appearance
Nuchal translucency thickness (if accepted after informed consent and trained/certified operator available)b
Face
Eyes with lensb
Nasal boneb
Normal profile/mandibleb
Intact lipsb
Spine
Vertebrae (longitudinal and axial)b
Intact overlying skinb
Chest
Symmetrical lung fields
No effusions or masses
Heart
Cardiac regular activity
Four symmetrical chambersb
Abdomen
Stomach present in left upper quadrant
Bladderb
Kidneysb
Abdominal wall
Normal cord insertion
No umbilical defects
Extremities
Four limbs each with three segments
Hands and feet with normal orientationb
Placenta
Size and texture
Cord
Three-vessel cordb
Prenatal Diagnosis of Congenital Anomalies by First-Trimester Ultrasound
Since the first reports demonstrating the feasibility of first trimester diagnosis of congenital anomalies in the late 1980s [1, 26, 27], mounting evidence indicates that accurate prenatal diagnosis of several of the more severe anomalies can be accomplished in the first trimester using high-resolution transvaginal ultrasonography [1, 2, 8, 23, 28]. Table 13.2 provides a summary of the studies published until 2014. These studies also provide evidence that, although early and accurate diagnosis of congenital anomalies is possible and allows early decision making, several anomalies may be missed if a second-trimester (or even a third trimester) scan is not performed. Examples of anomalies that can be missed by a first-trimester scan include vermian hypoplasia, agenesis of the corpus callosum, abnormalities of neuronal migration (e.g., lissencephaly, polymicrogyria, gray matter heterotopia), congenital lung anomalies, hypoplastic left heart, aortic and pulmonic valve stenosis, coarctation of the aorta, renal and bladder anomalies, gastrointestinal anomalies, as well as several skeletal anomalies that may manifest only later in pregnancy [7, 10, 12, 14, 25, 29–32].
Table 13.2
First-trimester detection rates for congenital anomalies
Author | Country | Year | N | Approach | Detected anomalies N (%) | Additional fetuses with anomalies detected >14 weeks (including second- and third-trimester scans and postnatally) N (%) | First-trimester detection rate (%) |
|---|---|---|---|---|---|---|---|
Rottem et al. [1] | Israel | 1989 | 141 | TV | 3 (2.12) | 0 | 100 |
Cullen et al. [27] | USA | 1990 | 622 | TV | 33 (5.31) | NA | NA |
Rottem and Bronshtein | Israel | 1990 | 1652 | TV | 40 (2.42) | 4 (0.24) | 90.9 |
Achiron and Tadmor [3] | Israel | 1991 | 800 | TA and. TV | 8 (1.00)a | 6 (0.75) | 57.1 |
Bonilla-Musolles | Spain | 1994 | 834 | TV | 27 (3.24) | 3 (0.36) | 90 |
Yagel et al. [29] | Israel | 1995 | 536 | TV | 42 (7.8) | 13 (2.4) | 76.4 |
D’Ottavio et al. [31] | Italy | 1995 | 4078 | TV | 54 (1.3) | 34 (0.83) | 61.4 |
Hernadi and Torocsik [30] | Hungary | 1997 | 3991 | TA and TV | 20 (0.41) | 29 (0.73) | 40.8 |
Economides et al. [63] | England | 1998 | 1632 | TA + TV | 11 (0.67) | 6 (0.37) | 64.7 |
Whitlow et al. [64] | England | 1999 | 6634 | TA + TV | 37 (0.56) | 55 (0.83) | 40.2 |
Guariglia and Rosatti [10] | Italy | 2000 | 3478 | TV | 33 (0.95) | 31 (0.89) | 51.6 |
Carvalho et al. [65] | Brazil | 2002 | 2853 | TA + TV | 29 (1.02) | 101 (3.54) | 22.3 |
den Hollander et al. [66] | Netherlands | 2002 | 101 | TA + TV | 9 (9) | 2 (2) | 81.8 |
Drysdale et al. [67] | England | 2002 | 984 | TA | 5 (0.51) | 25 (2.54) | 16.7 |
Taipale et al. [68] | Norway | 2004 | 4513 | TV | 6 (0.13) | 27 (0.59) | 18.2 |
Chen et al. [69] | Hong Kong | 2004 | 1609 | TA + TV | 14 (0.87) | 12 (74.6) | 53.8 |
Becker and Wegner [14] | Germany | 2006 | 3094 | TA + TV | 72 (2.36) | 14 (0.45) | 83.7 |
Saltvedt et al. [70] | Sweden | 2006 | 18,053 | TA | 74 (0.41) | 297 (1.64) | 20 |
Cedergren et al. [71] | Sweden | 2006 | 2708 | TA | 13 (0.48) | 19 (0.70) | 40.6 |
Dane et al. [72] | Turkey | 2007 | 1290 | TA + TV | 17 (1.32) | 7 (0.54) | 70.8 |
Chen et al. [69] | Hong Kong | 2008 | 3949 | TA + TV | 30 (0.76) | 33 (0.84) | 47.6 |
Oztekin et al. [73] | Turkey | 2009 | 1085 | TA and TV | 14 (1.29) | 7 (0.65) | 66.6 |
Ebrashy et al. [7] | Egypt | 2010 | 2876 | TA + TV | 21 (0.73) | 10 (0.35) | 67.7 |
Syngelaki et al. [28] | England | 2011 | 44,859 | TA + TV | 213 (0.48) | 275 (0.61) | 43.6 |
Iliescu et al. [11] | Romania and Greece | 2013 | 5472 | TA + TV | 67 (1.22) | 98 (1.05) | 41.1 |
Bromley et al. [12] | USA | 2014 | 9962 | TA + TV | 50 (0.50) | 130 (1.30) | 27.7 |
Goldstein et al. [21] | Israel | 2014 | 4467 | TA + TV | 33 (0.74) | 28 (1.04)b | 54.1 |
What Does 3D Ultrasound Add?
3DUS adds the possibility to obtain multiple planes of an anatomical structure from a 3D volume dataset. The elevation plane, in particular, which is perpendicular to the direction of the sound beam, is impossible to obtain using conventional 2DUS. This capability can be particularly advantageous during the first trimester, when manipulation of the vaginal probe is restricted and, therefore, the obtainable planes of section are limited [33]. Another potential benefit, provided that it can be proved beyond doubt that offline analysis of volume datasets has at least the same level of accuracy as real-time analysis of 2DUS images, is that embryonic exposure to ultrasound can be reduced, since volume acquisition takes only a few seconds and image processing and analysis can be performed offline [34].
Sonoembryology is the term that describes a detailed assessment of the live embryo in vivo by high-resolution transvaginal ultrasonography [33, 35, 36]. Initial publications on sonoembryology relied on images obtained by 2DUS. Since the original work describing the use of a specially designed high-resolution 3D transvaginal probe for reconstruction of small embryonic structures by Blaas et al. [37] in 1995, several investigators have reported on the use 3DUS for volumetric measurement [38, 39], assessment of normal embryonic development and early fetal anatomy [34, 40–46], as well as early prenatal diagnosis of congenital anomalies [42, 47–55].
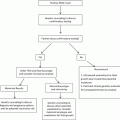
The best studied organ has been the embryonic brain, with initial studies focusing on volumetry and anatomy of cerebral brain vesicles [37, 38, 56]. Today, exquisite 3D images of the ventricular system can be obtained using commercially available equipment and inversion mode technology (Fig. 13.1), as reported by Kim et al. [42], who obtained 3DUS volumes of the embryonic and early fetal brain by transvaginal ultrasonography in 46 patients examined between 6 and 13 menstrual weeks. Inversion mode was used to reconstruct the early ventricular system. Appropriate reconstructions were possible only for volumes acquired between 7 and 12 weeks. Based on the experience with that work, the authors correctly diagnosed one case of alobar holoprosencephaly at 10 6/7 weeks and one case of early ventriculomegaly at 12 4/7 weeks.


Fig. 13.1
3D rendering of the early cerebral ventricles using inversion mode. P pontine flexure, D diencephalon (future third ventricle), M mesencephalon (future sylvian aqueduct), IR isthmus rhombencephali, RH rhombencephalic cavity
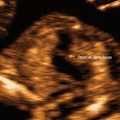
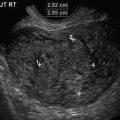
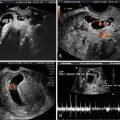
As it is natural to occur with any emerging technologies, several case reports and series have illustrated how 3DUS helped with specific diagnoses, mainly during the embryonic period [48–51, 57–60]. Among the most interesting are the early detection of a case of spina bifida at 9 weeks with exquisite detail of the defect demonstrated by 3D surface rendered images of the embryonic torso (Fig. 13.2) [49, 58–60], confident diagnoses of cyclopia [50] and proboscis [50, 51] by 3D multiplanar reconstruction at 9 2/7 weeks [50] and 10 6/7 weeks [51] in association with alobar holoprosencephaly, digital casts of the abnormal ventricular system in cases of holoprosencephaly as early as 9 2/7 weeks, conjoined twins at 9 [55] and 10 weeks [57], prune-belly syndrome [58], iniencephaly [45] and frontonasal malformation [61] at 11 weeks, and severe scoliosis associated with omphalocele [58] and encephalocele [45] at 12 weeks.