Fig. 16.1
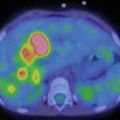
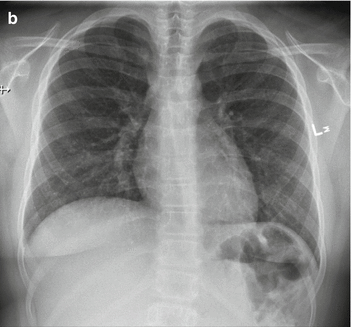
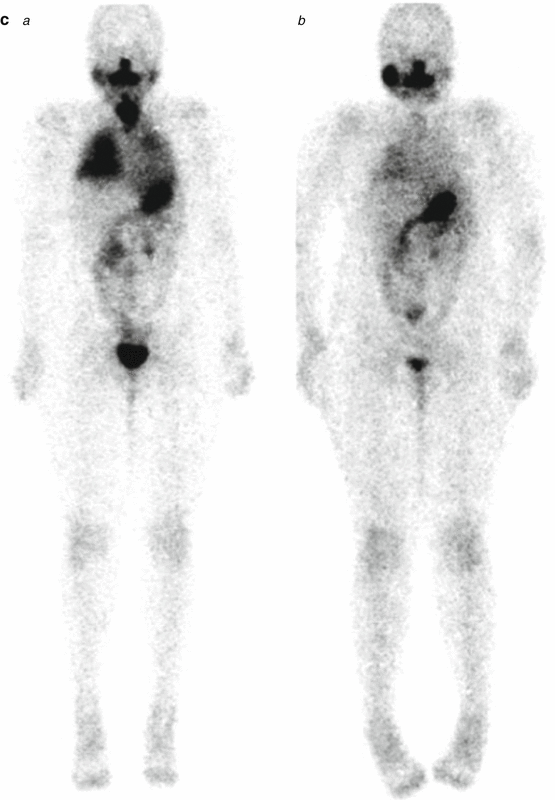
A 9-year-old female presented with a mass in L side of the neck. US (a): Mass L side isthmus: microcalcification, increased vascularity, and patchy heterogeneous echogenicity in remainder of gland. Abnormal lymph nodes bilaterally consistent with metastatic disease. Chest X-ray (b) was normal. Pre-RIT 123I WBS (c) shows marked RAI uptake throughout both lung fields and significant residual thyroid tissue and loco-regional disease within the neck (a). Follow-up 123I whole-body scan at 6 months post surgery (b) shows a good response to RIT (6.3 GBq) but with persisting disease diffusely throughout the lungs. She was treated with a further dose of 6.3 GBq 131I. Her Tg has fallen to 306 ug/L from a baseline of 642 ug/L. Note reduced uptake in the left salivary glands due to sialadenitis
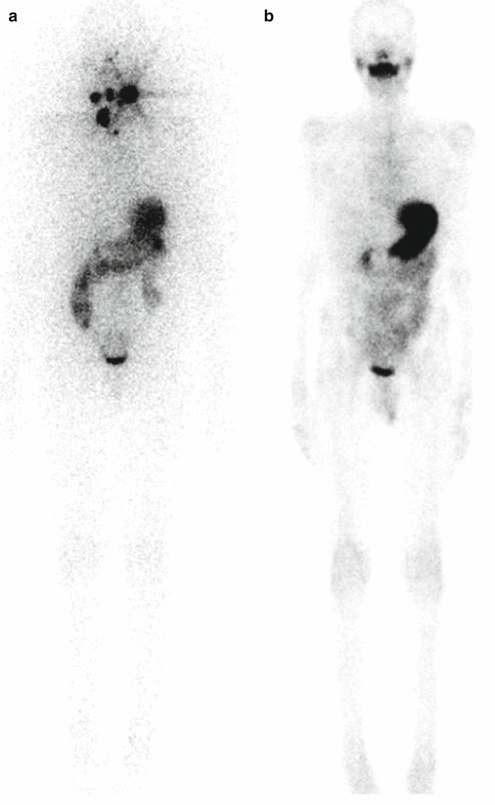
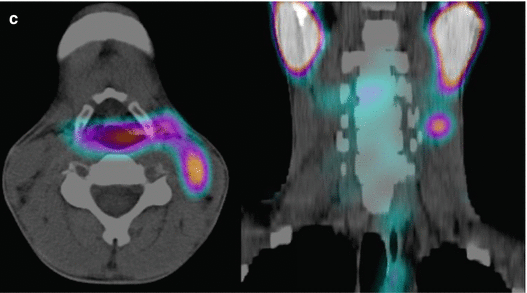
Fig. 16.2
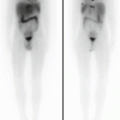
A 12-year-old male (weight 45 kg) presented with a dominant left thyroid nodule and palpable bilateral neck lymphadenopathy. Post thyroidectomy and extensive bilateral neck and mediastinal dissection, RAI therapy at a dose of 3.7 GBq was given. WBS shows extensive residual bilateral lymph node disease (a). At 6 months’ follow-up 123I WBS is normal (b). Tg after withdrawal of T4 (TSH 109 mU/L) was 6.4 ug/L, and US of the neck was normal. SPECT/CT (c) of the neck was performed and shows a focal increase consistent with recurrent disease in level II on the left side. This was confirmed at surgery
16.5 Surgery
The optimal surgical management in paediatric/adolescent DTC follows the surgical recommendations in adult DTC. Due to the high incidence of multifocal disease (42 %), loco-regional metastatic disease, distant metastases, and the higher recurrence rate in paediatric patients, near or total thyroidectomy is recommended [12, 25, 31, 34, 37]. Total thyroidectomy allows more accurate follow-up using serum Tg and 123I/131I whole-body scans. Near or total thyroidectomy results in a significantly lower recurrence rate in both adults and in children/adolescents. If nodal metastases are diagnosed preoperatively with ultrasound or biopsy, selective lymph node dissection is usually performed. Our institution and many other paediatric centres recommend that central compartment neck dissection should also be undertaken. This is particularly indicated if enlarged or abnormal nodes are detected by palpation or ultrasound. The main argument against total thyroidectomy is the risk of complications including hypoparathyroidism and laryngeal nerve damage. However if surgery is performed by experienced thyroid surgeons with paediatric expertise, there is no significant increase in complications [9, 12, 25, 31, 34, 37].
16.6 Radioiodine-131 Ablation and Therapy (RIT)
Surgery is usually followed by RIT, and this is a major therapeutic component of management in children and adults with DTC. The goal of the first RIT is for remnant ablation which facilitates initial staging and detection of recurrent disease in follow-up (serum Tg and 123I/131I WBS) and as adjuvant therapy with the aim of treating known residual disease and destroying microscopic disease (Figs. 16.1 and 16.2). There are large retrospective studies confirming a decrease in loco-regional recurrence and reduced overall specific mortality [6, 9, 30, 31, 39]. The National Thyroid Cancer Treatment Cooperative Study group showed after near total thyroidectomy followed by RIT and suppressive thyroid hormone (levothyroxine, LT4) therapy, there was significant improved overall survival in patients with NTCTCSG stage III and IV disease. There was some benefit in stage II disease but no impact in stage I disease [19]. Guidelines published by the ATA and the European Society for Medical Oncology agree in the use of RIT in high-risk adult patients and state that RIT is not necessary in unifocal papillary thyroid microcarcinomas (PTMC) without metastases, capsule invasion, and lack of aggressive histologies. In adults, low-risk patients have an excellent prognosis with no proven benefit from RIT, despite the risk stratification and methods used (AMES, MACIS). RIT may be recommended in low-risk patients for more specific follow-up using serum Tg measurements. RIT is also effective in the treatment of small residual tumours and metastases, which can occur in microcarcinoma [6, 10, 27]. Nodal metastases have been described in approximately 38 % of PTMC [17, 26]. Middendorp and Grünwald recommend that RIT should be undertaken routinely in DTC with few exceptions [24].
Even though the role of RIT in paediatric DTC continues to be controversial, the majority of paediatric centres managing DTC recommend RIT in all paediatric patients except possibly for stage I disease. RIT is strongly advocated by Chow et al. in children with DTC. They recommend RIT in paediatric patients if any of the following is present: tumour size >1 cm, cervical lymph node disease, extrathyroidal extension, residual postoperative disease in situ or distant metastases. Interestingly Chow et al. report only two children in their cohort with small <1 cm cancers. Both of these had metastatic disease: one had cervical lymph node involvement and the other pulmonary metastases. Local recurrence rates in children who did not receive RIT have been reported to be significantly higher (42 %) compared with children who received RIT (6 %). Pulmonary metastases developed in 20.8 % of these patients who did not have distant metastases at presentation and had total thyroidectomy but were not treated with RIT. A comparison cohort of 32 patients who had been treated with RIT did not develop pulmonary metastases. Differences were also found in outcomes for children treated between 1960 and 1986 compared with those treated between 1986 and 1997. Total thyroidectomy was less often performed prior to 1986 (67 % vs. 93.5 %), and these patients were less likely to receive RIT (44.8 % vs. 0 %). Children treated prior to 1986 had a higher rate of local recurrence (37.9 % vs. 3.2 %) and distant metastases (17.2 % vs. 0 %) [5]. Handkiewicz-Junak et al. reported 174 children treated with RIT after surgery. Multivariate analysis showed that total thyroidectomy and adjuvant RAI treatment independently decreased loco-regional recurrence risk. The overall consensus indicates that RIT significantly reduces recurrence of thyroid carcinoma even though this may take 20 or more years to occur [12, 28, 31]. A recent publication reported the higher likelihood of recurrence related to younger age group, conservative surgical management, no RIT and multifocal cancer [25]. For successful ablation, serum TSH must be elevated to >30 mU/L to achieve maximal RAI uptake. In children, this usually occurs faster than in adults and takes between 2 and 3 weeks of LT4 withdrawal. Hung and Sarlis advocate using l-triiodothyronine (LT3) immediately after surgery for 4 weeks and withdrawing this for a further 2 weeks prior to ablation [15]. Some centres are increasingly using LT4 supplement post surgery followed by recombinant human thyrotropin (rhTSH) stimulation for the RIT [23].
16.7 Pre-radioiodine Therapy Diagnostic Staging 123I/131I Whole-Body Scintigraphy
Pre-radioiodine therapy imaging with diagnostic RAI WBS is recommended in paediatric patients with DTC [5, 31]. At The Childrens’ Hospital at Westmead, Sydney, pre-RIT diagnostic WBS is undertaken in the majority of patients using 123I WBS with SPECT/CT (Figs. 16.2c and 16.3b). If 123I is not available, 131I diagnostic WBS should be performed. The pre therapy WBS enables the evaluation of residual thyroid tissue and extent of loco-regional and/or metastatic disease, whether there should be further completion surgery and determination of the appropriate RAI dose. This information allows for more informed communication regarding prognosis, risk of recurrence and overall survival, including discussion of risks and benefits relating to RAI therapy [36].
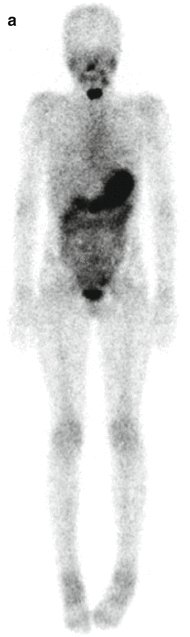
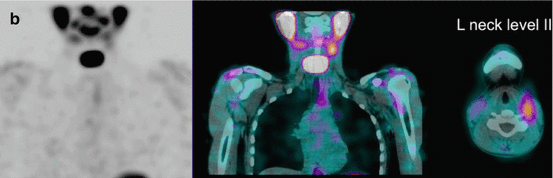
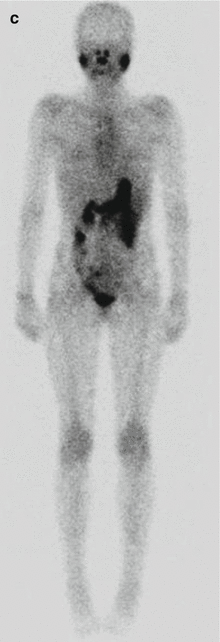
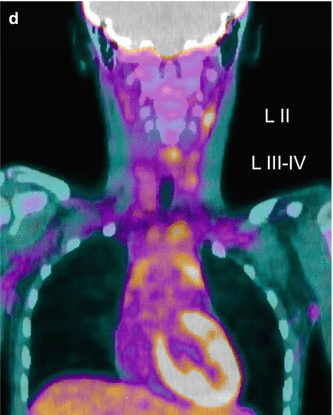




Fig. 16.3
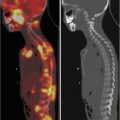
A 12-year-old female presented with a left-sided thyroid nodule. FNAB revealed Bethesda V papillary carcinoma diffuse sclerosing variant. The patient underwent total thyroidectomy and central compartment node dissection. The pre-RIT 123I WBS post surgery showed active uptake in the midline of the neck (a). SPECT/CT showed a faint increase in a level II lymph node on the left side (MIP, coronal, axial, co-registered) 123I scan (b). At 6 months’ follow-up, her 123I scan was normal (c). However her Tg was elevated at 81.5 ug/L. Ultrasound was normal. 18 F-FDG PET/CT (d) was performed after withdrawal of T4, and this revealed multiple focal areas of uptake in the left neck levels II and II–IV and an area in the retrotracheal region (co-registered PET/CT coronal view). Extensive left neck dissection was performed and 15 nodes were removed. Three nodes were positive for thyroid carcinoma. Genetic testing revealed an ALK mutation which indicates a more aggressive disease
16.8 Risk-Adapted Management or Ongoing Risk Stratification
The ATA in 2009 proposed a new classification for an estimate of recurrence of disease by a classification of the probability of recurrence as low, intermediate or high [6, 39]. Other parameters which are important for staging are available after surgery and if RAI ablation or RIT has occurred. The variables include tumour histology, evidence of vascular invasion, multifocal disease, involvement of regional lymph nodes and any evidence of systemic metastases [3, 11, 39]. This risk-adapted approach requires risk estimates that change over time based on response to therapy and the course of the disease. Tuttle et al. reported 588 adult patients with DTC who underwent total thyroidectomy and RIT who were stratified into low, intermediate and high risk according to ATA categories. Persistent structural disease or recurrence was identified in 3 % of low-risk, 21 % of intermediate-risk and 68 % of high-risk patients. Re-stratification in the first 2 years of follow-up reduced the likelihood of finding persistent structural or recurrence in 2 % of low-risk, 2 % of intermediate-risk and 14 % of high-risk disease. This demonstrated an excellent response to therapy with Tg <1 ug/L without evidence of structural disease. Conversely an incomplete response to initial therapy (suppressed Tg >1 ug/L, stimulated Tg >10 ug/L), rising Tg or structural disease identified within the first 2 years increased the likelihood of structural disease or recurrence in 13 % of low-risk, 41 % of intermediate-risk and 79 % of high-risk disease [39]. This data has been confirmed by Castagna et al. [3]. This method of risk stratification has not been reported in paediatric DTC; however the data on overall survival and mortality related to DTC in paediatric patients would suggest that this risk stratification estimation also applies to paediatric and young adolescent patients.
16.9 Recombinant Thyrotropin (rhTSH) Use for Diagnostic RAI WBS and Therapy
Thyroid hormone withdrawal may not be tolerated well. However most children and adolescents usually raise their TSH more rapidly than adults after withdrawal of LT4. Serum TSH may be elevated by using recombinant thyrotropin (rhTSH). Patients are reported to feel better with rhTSH compared to the withdrawal method. The radiation dose is approximately 30 % less [23, 28, 31]. Iorcansky et al. reported in children and adolescents a comparison between withdrawal and rhTSH. TSH levels after rhTSH stimulation (134 ± 75 mIU/L) were not significantly different to TSH levels following withdrawal (188 ± 118 mIU/L) [16]. Luster et al. confirmed this in a multicenter trial with the application of rhTSH in 100 DTC patients aged between 4.9 and 18 years. Ninety-two percent of these patients received the adult dose of 0.9 mg IM for 2 consecutive days and 34 % also combined with LT4 withdrawal for <7 days. No clinical adverse events occurred in 88 % of the rhTSH courses. Nausea (5 %) and vomiting (3 %) were the most common side effects. The peak concentration of TSH was >25 mU/L in 98 % of cases [22]. A benefit of using rhTSH in paediatric patients is the reduced radiation exposure; however, rhTSH is not approved by drug regulatory agencies in the USA and Europe [31].
16.10 Paediatric Dose of RAI
There is no consensus on the appropriate dose of RAI for ablation and adjuvant therapy in paediatric and adolescent patients. Studies in adults using 131I for remnant ablation in low-risk patients have recommended doses at 30 mCi (1.11 GBq) and compared this to higher doses of 100 mCi (3.7 GBq) both with withdrawal method and using rhTSH. No significant differences in recurrence and outcome were found [4]. In addition most centres recommend low-iodine diets for 1–2 weeks prior to RAI treatment, and the patients should not receive iodinated contrast for imaging studies.
Dose calculation of 131I is based on three methods: ablation, adjuvant therapy and dosimetry. The empiric method in adults is based on fixed activities usually 100 mCi (3.7 GBq) for ablation and neck loco-regional disease, 150 mCi (5.5 GBq) for pulmonary metastases and 200 mCi (7.4 GBq) for bone and other metastases. The majority of paediatric centres treating DTC in childhood and adolescence use the empiric method, and currently, this is weight based or surface area adjusted to the adult dose of 70 kg [15, 31]. Parisi and Mankoff suggest adult doses 100 mCi (3.7 GBq) for low-risk patients, 150–175 mCi (5.5–6.5 GBq) for patients with loco-regional disease and doses up to 200 mCi (7.4 GBq) for high-risk patients with large tumours, capsular invasion, extrathyroidal spread, extensive nodal disease or distant metastases. The dose is adjusted to weight, and some selected patients may have the dose based on dosimetry [28]. Our institution follows this empiric method. Other factors that should be taken into account are previous radiation therapy or if the patient has had cumulative treatments with 131I and this is approaching 600 mCi (22.8 GBq) [15].
131I activity based on dosimetry that is as high as safely administrable (AHASA) showed that blood doses greater than 2 Gy, whole body retention of more than 120 mCi at 48 h or 80 mCi retained by the lungs at 24 h were associated with bone marrow suppression and lung fibrosis [2]. Recently, Verburg et al. reported AHASA levels for children and recommended for treatment of distant metastases. Activities up to 5 mCi/kg (200 MBq/kg) were found to be the highest safe limit. For initial ablation, even if pulmonary metastases may be present, the authors state that at least 100 MBq/kg can be administered safely [40]. Dosimetry allows dosing on patient tolerance; however the techniques are very complex and time-consuming and take up to 4–5 days [20]. There is no data in the literature indicating this method is better in regard to survival and recurrence free survival than the empiric method of dose of RAI but may provide data which could become helpful in the future to optimise the benefit-radiation risk ratio particularly in patients with diffuse pulmonary metastases.
16.11 Risks of RIT
1.
Second primary malignancy
2.
Reproductive issues
3.
Pulmonary fibrosis
4.
Others
16.11.1 Second Primary Malignancy (SPM)
Initial studies of SEER database of 30,000 adults treated with RIT revealed no effects of RIT on SPM risk. However recent review of this data suggests a small carcinogenic effect with increased rates of haematological and solid SPM. An increased risk of solid tumours and leukaemia was found with activities >200 mCi (7.4 GBq) and 100 mCi (3.7 GBq). No effects were found with lower activities [31, 32]. Garsi et al. presented follow-up data on 11,007 European patients with DTC. Patients >20 year of age had a risk of SPM of about 25 % higher than the general population; however, the risk was not related to RIT for most patients but to having DTC because the risk without RIT was also 25 %. This suggests a genetic predisposition to SPM. An RAI-related risk of SPM was seen when the cumulative dose of 131I was >200 mCi (7.4 GBq). Rubino et al. evaluated the European cohort for patients < 20 years of age and found no evidence of increased risk of SPM after treatment of DTC in children [31]. Sawka et al. reported a systematic review and meta-analysis of the literature. The relative risk (RR) of SPMs in survivors who were treated with RIT was increased with an RR of 1.19 compared to patients not treated with RIT using a minimum latency period of 2–3 years after diagnosis. The RR for leukaemia was increased at 2.5 %. The absolute risks were calculated at approximately 1 % for SPM and 0.4 % for leukaemia. The authors concluded that the risk of SPMs in thyroid cancer survivors treated with RIT is slightly increased compared with those not treated with RIT [35]. Reiners et al. reported on outcomes on 234 high-risk post Chernobyl cases of children and adolescents with DTC. The median follow-up was 11.3 years. Distant metastases were found in 100 patients. No haematological or solid malignancies were found [29]. Similar data was reported by Michailovic et al. in a long-term follow-up of 51 paediatric patients with no increased incidence of reproductive issues or SPM [25]. However as paediatric patients and adolescents are younger when receiving RIT and have a much longer life expectancy, it should be considered that there is an unknown but probable increased risk of SPM in these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree