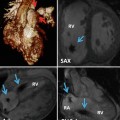
Fig. 22.1
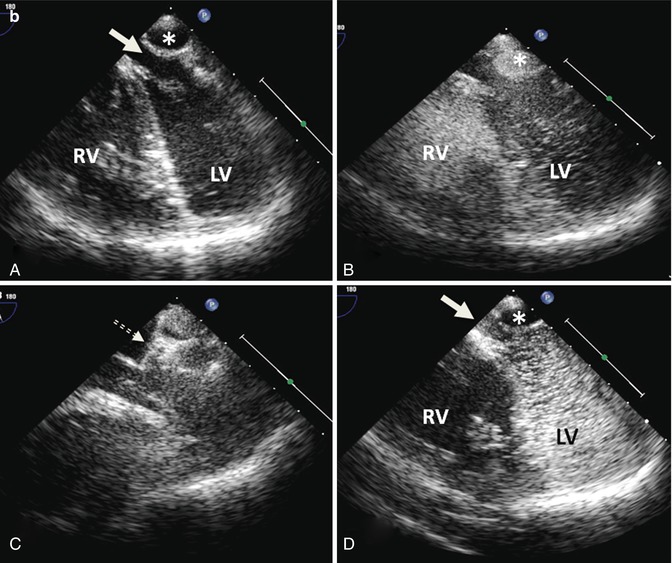
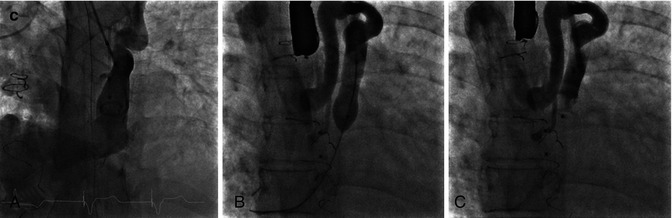
(a) CTA from a patient with a Mustard procedure for transposition of the great arteries. There is a large left-sided SVC, which drains to the coronary sinus (thick arrows). The right-sided SVC (thin arrow) is baffled to the subpulmonic (morphological left) ventricle. The coronary sinus (CS) has been incorporated into the pulmonary venous side of the baffle (systemic atrium), giving rise to a right-to-left shunt (b) Series of transesophageal echocardiography images in the same patient. (A) Demonstrates a 4-chamber view with systemic right superior vena cava flow baffled (white arrow) to the left (subpulmonic) ventricle (LV). The pulmonary venous atrium is indicated by the asterisk. (B) Bubble contrast injection in the left arm demonstrates preferential filling of the pulmonary venous atrium and right (subaortic) ventricle (RV) consistent with right-to-left shunt. (C) Demonstrates the Amplatzer duct occluder in position in the left SVC (dashed arrow). (D) Repeat bubble contrast injection in the left arm with no further filling of the pulmonary venous circuit/RV indicating abolition of the right-to-left shunt. (c) Series of angiographic images outlining the angiographic findings. (A) Left SVC draining to the systemic venous atrium. (B) Balloon occlusion of the left SVC with subsequent venous drainage to the right SVC. (C) Final angiogram following release of the Amplatzer duct occluder
Careful attention should be given to the type of anesthesia required and access to a high-risk preanesthesia clinic is helpful. Decision regarding the type of anesthesia may reflect the complexity of the intervention or the need for and type of intraprocedural imaging (intracardiac vs transesophageal echocardiography). In simpler cases or those who are high-risk patients, general anesthesia may be avoided with the use of intracardiac echocardiography (ICE). ICE provides detailed images of all intracardiac structures including the pulmonary valve following transcatheter pulmonary valve replacement when residual catheters across the valve required for angiography may exaggerate the degree of pulmonary incompetence (Fig. 22.2).
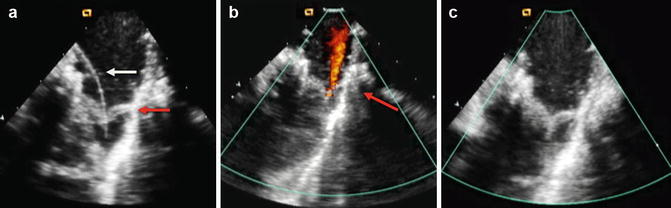

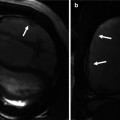
Fig. 22.2
Series of intracardiac echocardiogram images following transcatheter pulmonary valve replacement. (a) Following valve deployment (red arrow), there is a wire (white arrow) still across the valve. (b) Mild pulmonary valve regurgitation (red arrow) is seen as a consequence of the wire distorting the valve. This was also seen on angiography with the catheter across the valve. (c) Once the wire is removed, the ICE images clearly demonstrate no appreciable pulmonary regurgitation
Collaboration between pediatric and coronary interventionalists should be encouraged as varying experiences bring fresh perspective particularly in the setting of coronary artery work with coronary artery fistulae, when the need for rapid intervention to the coronary artery may be required (Fig. 22.3). Honesty is required regarding level of comfort with each specific procedure, and expectation and pride should never impact upon optimal patient care.
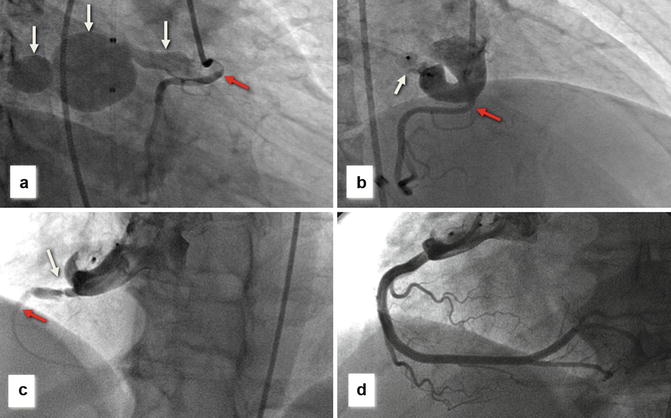

Fig. 22.3
(a) Right coronary angiogram demonstrating large bilobed coronary artery fistula (white arrows) originating from the proximal right coronary artery (RCA) (red arrow) and draining to the right atrium (white arrow). (b) Following deployment of an Amplatzer muscular occluder (white arrow), there is no residual distal flow to the fistula and the RCA fills well (red arrow). (c) ST elevation was noted on a routine post-procedural ECG with repeat right coronary angiography demonstrating a discrete narrowing in the RCA (probable intimal flap – white, arrow) with severely attenuated distal filling (red arrow). (d) Final RCA angiogram following implantation of three coronary stents demonstrates excellent rehabilitation of the RCA
Thus the adult congenital interventionalist is often required to be the coordinator for a number of subspecialists to focus detailed imaging review and collaborate with colleagues who provide specialist input so as to optimize outcomes from complex and varied interventions.
Defect and Vascular Occlusion
Transcatheter Atrial Septal Defect Closure
Atrial septal defect (ASD) accounts for 25–30 % of congenital heart defects that are diagnosed in adulthood. Clinical guidelines for closure in adults are published [2] and class one indications (level of evidence B) support closure in the presence of right atrial and right ventricular enlargement with or without symptoms. The presence or absence of symptoms has clinical relevance to outcomes, although most long-term follow-up studies evaluate surgical closure. Historical data indicate that outcomes after ASD closure in symptomatic adults <25 years old are similar to age-matched controls; however, patients >40 years old undergoing closure have lower survival than controls [4]. That said, closure in symptomatic adults >40 years still provides a survival advantage [4, 5]. In contrast closure in asymptomatic adults reduces morbidity but not mortality [6].
Randomized comparisons of transcatheter and surgical closure of ASDs are lacking; however, a non-randomized comparison of 596 patients undergoing ASD closure (442 surgical and 154 transcatheter) revealed a periprocedural complication rate of 7 % in the transcatheter group versus 24 % in the surgical group. This has been corroborated in older patients, most recently in a report evaluating clinical outcomes after device (81 %) or surgical (19 %) ASD closure in 67 patients with a mean age at closure of 68 years [7]. At follow-up of 3.3 years, right ventricular end-diastolic dimensions, right ventricular function, and New York Heart Association functional class showed significant improvement in both groups; however, major complications were higher for the surgical group (23 % vs 7 %). Major procedural complications during/following transcatheter closure included device embolization and atrial erosion with a large contemporary multi-institutional report revealing a major adverse event rate of 1.1 % [8]. A large database analysis demonstrated a risk of device embolization of approximately 0.6 % [9]. Percutaneous retrieval is often possible; however if surgical retrieval is required, the mortality rate for the procedure increases to approximately 2 % [10]. The exact mechanisms leading to atrial erosion are unclear; however, device oversizing and inadequate anterosuperior rims have been implicated with an overall reported incidence with the Amplatzer septal occluder (St Jude Medical, St Paul, MN) between 0.1 and 0.2 % [11]. CT imaging has been used in this setting to evaluate particularly larger device position in relation to the surrounding cardiac structures [12] and has been useful in less clear-cut cases to determine if one of the discs of the ASD device has migrated from within the atrium (Fig. 22.4). Erosion has not been reported with the only other FDA-approved device for ASD closure, the Gore septal occluder (Gore and Associates, Flagstaff, AZ); however, device fracture rates of up to 7 % have been seen especially with the largest (35 mm) occluder. Future endeavors are likely to concentrate on biodegradable implants; however, although initial clinical experience with a predominantly bioabsorbable device was promising [13], this has not reached widespread acceptance as yet.
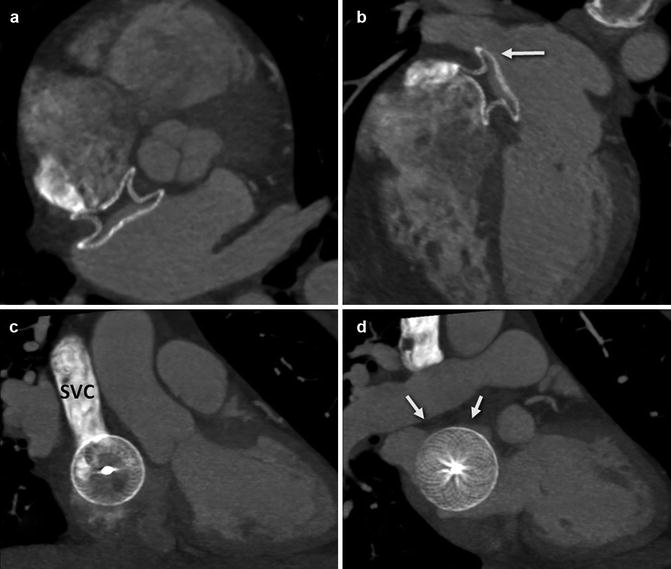

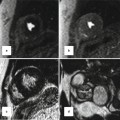
Fig. 22.4
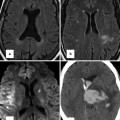
Series of CT images in an older patient who underwent transcatheter ASD closure with an Amplatzer septal occluder. There was a small pericardial effusion by echocardiography the day following defect closure, and CT was performed to evaluate the position of the device in relation to the other cardiac structures. (a) Axial CT maximal intensity projection (MIP) image demonstrates the occluder device in place approaching the aorta posterolaterally; with no evidence, it has moved beyond the borders of the atrial wall. (b) Four-chamber view demonstrating the relationship of the device to the right upper pulmonary vein (white arrow). (c) Demonstrates en face view of the right atrial disc and its relationship to the aorta. There is contrast in the superior vena cava (SVC) superiorly. (d) Same projection demonstrates the relationship of the left atrial disc to the roof of the atrium (arrows)
Transcatheter Ventricular Septal Defect Closure
Most clinically relevant VSDs are closed in childhood; however, hemodynamically, significant shunts [defined as a Qp/Qs >1.5:1 in adults [2]] may be only become clinically relevant in early adulthood. Reports outlining transcatheter closure exclusively in adults have been published [14, 15]. Indications for closure (Table 22.1) in these studies included symptoms and/or ventricular dilatation in 40–60 % of patients. Other common indications included pulmonary hypertension and endocarditis. Aortic valve prolapse leading to aortic regurgitation may be seen with small subaortic VSDs secondary to the Venturi effect in essence “sucking,” most commonly, the right coronary cusp inferiorly towards the VSD, and surgery is usually preferred in these instances as device placement may impinge upon the aortic leaflets worsening the aortic regurgitation. Transcatheter closure of perimembranous defects has been halted in the USA due to significant concerns regarding complete heart block [approximately 5 % in the European registry series [16] and 3 % in the US trial [17]] following device placement. Recovery of atrioventricular block has been reported with early treatment with steroid [18] or early surgical removal of the membranous occluder [19]; comparable rates of complete heart block following surgical closure in younger patients have been reported at less than 1 % [20]. Newer devices are being evaluated with design modifications to reduce radial force on the conduction tissue and potential for heart block. Overall, reported complication rates have varied ranging from 6.5 % in the European registry [16] to 10.7 % in the US perimembranous registry including device embolization, cardiac perforation, stroke, and two deaths [17]. Exclusion criteria have also been cited and include inadequate distance for device placement (usually 4 mm) between the VSDs and either the atrioventricular or semilunar valves, active sepsis, or pulmonary vascular resistance >7 indexed Wood units.
Table 22.1
Indications for interventional procedures
Intervention | Indication |
|---|---|
Transcatheter VSD closure | Qp/Qs ≥2.0 and clinical evidence of LV volume overload (class I) |
Qp/Qs >1.5 with pulmonary artery pressure less than two thirds of systemic pressure and PVR less than two thirds of systemic vascular resistance (class IIa) | |
Device closure is a class IIb recommendation [2] | |
Balloon pulmonary valvuloplasty | Symptoms or asymptomatic with peak Doppler gradient >60 mmHg/mean Doppler gradient >40 mmHg |
Balloon aortic valvuloplasty | Symptoms and peak-to-peak gradient catheter gradient >50 mmHg. Asymptomatic patient: peak-to-peak gradient catheter gradient >60 mmHg |
Balloon angioplasty/stenting for CoA | Transcatheter systolic coarctation gradient of >20 mmHg or <20 mmHg in the presence of significant collateral vessels and suitable angiographic anatomy, irrespective of patient age, as well as in patients with univentricular heart or with significant ventricular dysfunction |
Pulmonary artery stenting | Gradient >20 mmHg across the stenosis area, elevation of the RV or proximal main pulmonary artery pressure to greater than one half to two thirds of systemic pressure, or relative flow discrepancy between the two lungs of 35 %/65 % or worse |
Persistent Ductus Arteriosus
There are closure-related issues in regard to the persistent arterial duct specific to the adult population including calcification of ductal tissue and an increased likelihood of pulmonary hypertension [21]. Change in the orientation of the arterial duct with age may lead to a more horizontal entry into the pulmonary artery, and this may also have implications for transcatheter approach and device choice. The most widely reported device is the Amplatzer duct occluder (St Jude Medical); however, the symmetrical design of the Amplatzer muscular occluder may be more appropriate particularly when there is concern regarding pulmonary hypertension [21]. Closure rates are well over 90 % with low procedural morbidity [22].
Coronary Artery Fistulae
Congenital coronary artery fistulae are rare with a reported incidence of approximately 0.5 %. Most are small, have no clinical implication, and may regress spontaneously; however, larger fistulae may lead to significant left-to-right shunting and symptoms in adult life. Pre-procedural advanced imaging may be beneficial in determining the degree and extent of coronary dilation assisting with procedural planning and supporting decision making (Fig. 22.5). Both surgical and transcatheter closure have been described. Concerns regarding propagation of clot and acute myocardial infarction exist following device closure. A recent follow-up report of 76 patients over 50 years of age, assessing both transcatheter (n = 44) and surgical management, demonstrated major complications in 15 % of patients, the majority of these being myocardial infarction [23]. This series did not carry out adequate serial imaging of the coronary arteries and it may be that more subtle abnormalities remain undetected. Predictors of adverse outcomes included drainage to the coronary sinus, older age at diagnosis, systemic hypertension, and hyperlipidemia. Collaboration with coronary interventionalists may be advised as acute coronary complications may occur (Fig. 22.3). We advocate CT imaging as a tool to follow these patients. Attention to the coronary arteries is extremely important since many of these patients post closure of the fistulae may have abnormal coronary arteries.
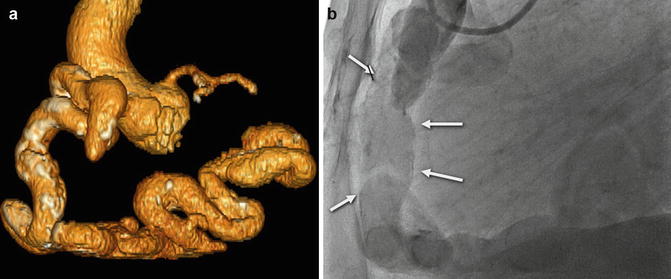

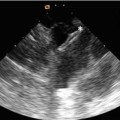
Fig. 22.5
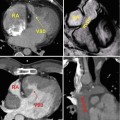
(a) Volume-rendered CT coronary angiogram from a 66-year-old with high output congestive heart failure. There is a large dilated, calcified, and tortuous RCA. (b) RCA angiography confirmed the large dilated RCA (white arrows), and due to concomitant left coronary artery disease, he underwent surgical bypass grafting along with tricuspid valve repair. The CT images were extremely useful in guiding the decision making regarding optimal therapy in this patient
Ruptured Sinus of Valsalva Aneurysms
Sinuses of Valsalva aneurysms (SoVA) are rare however are prone to rupture usually in adult life and lead to significant mortality within 2–4 years. Congenital SoVAs arise due to a deficiency of elastic fibers in the aortic media leading to progressive dilatation over time. The right sinus of Valsalva is affected in up to 85 % of cases with the left sinus almost never affected. Usually the rupture occurs into the right atrium or ventricle leading to significant left-to-right shunt and congestive cardiac failure. Although historically surgery was the mainstay of treatment, transcatheter closure has established itself as a less-invasive alternative. Most reported cases to date have described use of the Amplatzer duct occluder (St Jude Medical) [24, 25]. Although most reports are limited to small numbers, successful occlusion with clinical improvement has been achieved in over 90 % of cases [24, 25]. Longer-term outcomes are as yet unknown.
Other Lesions
In patients with inadequate pulmonary blood flow (i.e., tetralogy of Fallot), aortopulmonary collaterals may develop, leading to left-to-right shunting and/or hemoptysis. Coil occlusion has been reported successfully for many years with good occlusion and low complication rates. In patients with chronic hypoxemia however, these lesions may be complex and progressive and coiling may in fact limit distal access to further developing lesions. More recently deployment of microspheres and copolymers of ethylene vinyl alcohol have been reported [26], providing a more complete distal occlusion of all branching vessels.
Intrapulmonary arteriovenous malformations (AVMs) leading to hypoxemia or risk of systemic embolization may occur as an isolated congenital malformation or as part of a systemic syndrome (Osler-Weber-Rendu syndrome). Successful transcatheter occlusion has been reported [27]. More diffuse micro-AVMs may be seen in patients with complex congenital palliative surgeries where hepatic blood flow is excluded from the pulmonary microvasculature, thought to occur secondary to an as yet unidentified “hepatic factor.” These AVMs are usually not amenable to transcatheter treatment but may regress with restoration of hepatic venous blood flow to the pulmonary circuit.
Other collateral vessels encountered in patients with congenital heart disease include large venovenous collaterals in the setting of a single-ventricle circulation, particularly recanalization of a left superior vena cava in patients with a superior cavo-pulmonary anastomosis or total cavo-pulmonary connection. Device occlusion has also been described for fenestrated inferior cavo-pulmonary connections, surgically created to decompress the pulmonary vascular bed in patients with marginal pulmonary vascular resistance in the setting of single-ventricle palliation, with good success. Test occlusion is usually carried out prior to definitive device closure to ensure cardiac output is not significantly compromised [28].
Angioplasty and Valvuloplasty
Balloon Pulmonary Valvuloplasty
Pulmonary valvuloplasty for congenital pulmonary valve stenosis has demonstrated excellent outcomes with low complication rates over the past 30 years. Indications in the adult population include a peak instantaneous Doppler gradient >60 mmHg (mean >40 mmHg); however, intervention at lower gradients may be acceptable in the presence of symptoms [2]. Balloon-to-annulus ratios of up to 1.4:1 have been recommended in the past; however, due to development of moderate to severe pulmonary insufficiency in younger patients, a more conservative approach has been advocated [balloon/annulus ratio of 1.2:1]. With larger annulus diameters in adults, a double-balloon technique may be required to achieve effective dilation of the valve. Long-term follow-up data is available in adults [29]. Significant decreases in peak pulmonary gradients were achieved in 90 adults with congenital pulmonary valvular stenosis undergoing balloon dilation, with regression in pulmonary infundibular stenosis and tricuspid regurgitation over time, following decompression of the right ventricle. Thirty percent of the cohort developed mild pulmonary regurgitation; however, this was nonprogressive in all but two cases which is less than the majority of reported cases following surgical valvotomy. Balloon dilation has also been employed in younger patients for both subvalvular and supravalvular stenoses; however, responses to treatment in this setting have been less encouraging.
Balloon Aortic Valvuloplasty
Congenital valvular aortic stenosis accounts for approximately 5 % of congenital heart defects and may be associated with genetic defects such as Turner’s syndrome. In milder cases progression of stenosis may occur through childhood with one study demonstrating 17 % of children requiring intervention at a median age of 10.5 years, following initial diagnosis at a median age of 2 years [30]. Indications for intervention are outlined in Table 22.1. The standard approach in older patients has been retrograde via a transfemoral approach. However reports in adults have suggested that crossing the aortic valve in the same direction as blood flow (via a transeptal venous approach) may reduce the risk of valve leaflet perforation and leaflet avulsion during inflation, thus limiting some of the major precipitants for significant aortic valve damage during valvuloplasty [31




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree