Transesophageal Echocardiography for Coronary Revascularization
INTRODUCTION
TEE provides an astutely trained clinician with essential information which can significantly influence clinical management and improve patient outcome in both cardiac and noncardiac procedures (1,2). In 2011, the American Society of Anesthesiologists/Society of Cardiovascular Anesthesiologists (SCA) Task Force published updated practice guidelines and classified hemodynamic instability as a Category I indication for the use of TEE and the use in coronary revascularization for patients with poor ventricular function as category IIa (3). The use of TEE in routine coronary artery surgery is also now considered IIa (3,4). The European Association of Echocardiography (EAE) and the SCA recommend that TEE should be used for both elective and emergency cardiac surgery unless contraindicated (3,4).
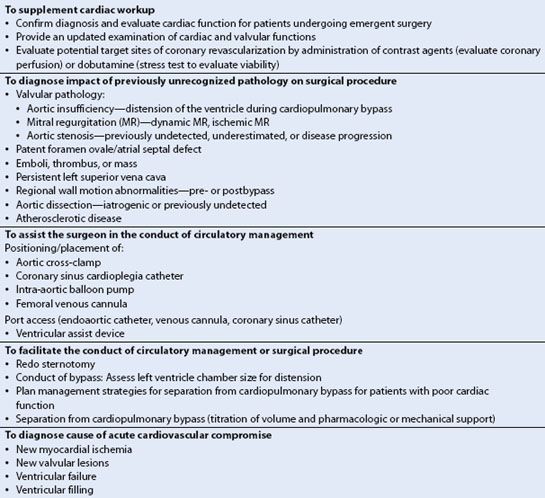
TEE provides an enormous amount of information during cardiac surgery that may directly change surgical case management in up to 13% of cases prebypass and in 6% postbypass (5–8) as well as anesthetic management in approximately 50% of coronary artery bypass graft (CABG) patients (Table 15.1) (5). The new information provided and subsequent interventions have been shown to be cost-effective (9). Skinner and Klein have also shown that preoperative studies may not accurately reflect patient pathology due to inadequacies of transthoracic echo, an inaccurate or incomplete report, and/or disease progression (7,10).
TEE provides a rational basis for decision-making during weaning from bypass by being able to continuously assess cardiac function, cardiac output, volume replacement, inotropic and vasoactive therapies as well as guide placement of intra-aortic balloon pumps (IABPs). TEE allows immediate assessment of the adequacy of revascularization by examination of regional wall motion abnormalities (RWMAs) both during on-pump and off-pump coronary artery procedures. Furthermore, TEE has proved reliable and comparable with the pulmonary artery catheter (PAC) and standard thermodilution techniques for monitoring and quantifying cardiac output.
The increasing age and comorbidity of patients undergoing coronary revascularization procedures make TEE an invaluable part of our monitoring armamentarium. For example, it allows us to optimally fluid resuscitate the patient and note the corresponding value of central venous pressure (CVP) or pulmonary artery pressure which can then be used in the intensive care unit. This is particularly important in hypertensive patients and those with ventricular systolic or diastolic dysfunction, in which venous pressure does not accurately reflect intracardiac volume (11,12).
SAFETY AND COMPLICATIONS
The risk of major complications, notably perforation of the esophagus, is small at 0.01% to 0.02% as assessed by a number of large studies. Minor complications such as sore throat and odynophagia are common but can be minimized by using a laryngoscope for placement (13–15). Antibiotic prophylaxis is not indicated for the majority of cases, though in high-risk cases, for example, ventilated patients with prosthetic valves on intensive care where oropharyngeal transfer of bacteria can occur, prophylaxis may be advisable. In every case, there is a balance of risks to be assessed with absolute and relative contraindications. In many enthusiasts’ eyes, the only absolute contraindications to a TEE will be an esophagectomy, recent esophageal surgery, or significant esophageal pathology.
TABLE 15.1 Role of TEE in Patients Undergoing CABG
PREBYPASS EXAMINATION
The prebypass intraoperative examination should target the pathology at hand, be performed in a systematic manner, and be organized into a comprehensive study. In contrast, the postcardiopulmonary bypass (CPB) examination is usually targeted toward assessing the results of the intervention and troubleshooting of possible complications. Preoperatively digital echo loops should be recorded to review and compare with examination findings during the postbypass period. A TEE examination should always include a written report for the patient’s records and a discussion of the findings with the cardiac surgeon.
Assessment of Ventricular Function
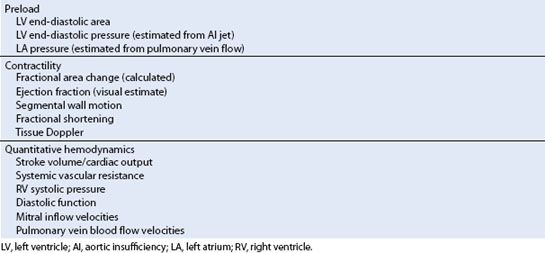
Traditionally, LV filling and cardiac output have been quantified by using a PAC. Intraoperative TEE often supplements or replaces these measures in current practice. Real-time echo images allow accurate qualitative evaluation of cardiac output, quantification of ventricular and valvular functions, intraoperative hemodynamic assessment, and most importantly contractility. Hypovolemia or severe LV dysfunction is easy to detect but various degrees of RWMAs can often be subtle as well as subjective and operator dependent. The standard views used to evaluate global LV function or regional ischemia (16) are discussed in Chapter 4. Table 15.2 summarizes the other TEE tools available to access cardiac function.
TABLE 15.2 Assessment of Cardiac Function by TEE
New Advances in Intraoperative Assessment of Global and Regional Cardiac Function
The automated border detection technique of acoustic quantification (AQ) introduced in the mid-90s to obtain area changes has been a step toward real-time, quantitative assessment of ventricular filling and ejection (Video 15.1). Tissue Doppler imaging (TDI) and real-time three-dimensional (RT3D) echocardiography echo may bring us closer to accurately measuring and quantifying myocardial function (17). However, because of angle dependency, fast-changing hemodynamic conditions, and lack of accurate validation studies, intraoperative TDI has never found its way into routine intraoperative TEE practice and has been replaced by other techniques to display myocardial deformation.
RT3D is useful to search for myocardial ischemia in high-risk surgical patients, in addition to assessing LV volumes and ejection fraction (EF) (18). Multiple slices from the apex to the base can now be displayed simultaneously (19,20). A computer-generated endocardial cast can graphically display the timing of contraction of each of the 17 segments to detect delays that may represent myocardial ischemia (Figs. 15.1 and Figs. 15.2). Evaluation of RWMAs with RT3D is still qualitative and therefore subject to observer bias. Initial studies of RT3D using dobutamine stress echo suggest that these multisliced displays are highly specific for the detection of angiographically confirmed ischemic myocardial regions (20). There are other new quantitative methods of detecting myocardial ischemic deformation. For example, strain measured by speckle tracking uses two-dimensional (2D) images and analyzes the movement of stable acoustic markers (speckles) between frames (19–21).
Monitoring of Ischemia
Visualizing the Aortic Root, Coronary Arteries, and Coronary Blood Flow
TEE imaging of the coronaries is discussed in detail in Chapter 4. As the aortic root runs oblique to the sagittal plane, the origin of the LCA is interrogated in a more superior (cephalad) plane than the RCA in TEE SAX views. TEE visualizes approximately 70% to 88% of the left and 25% to 50% of the right coronary artery ostia (22). Although difficult to quantify, flow can often be visualized with TEE in more distal branches of the larger coronary arteries, that is, the circumflex coronary artery in the atrioventricular groove (23) (Fig. 15.3, Video 15.2). It is important to recognize, both for ischemia detection and revascularization procedures, the patient variability in the coronary blood supply to the various myocardial segments (Figure 4.2).
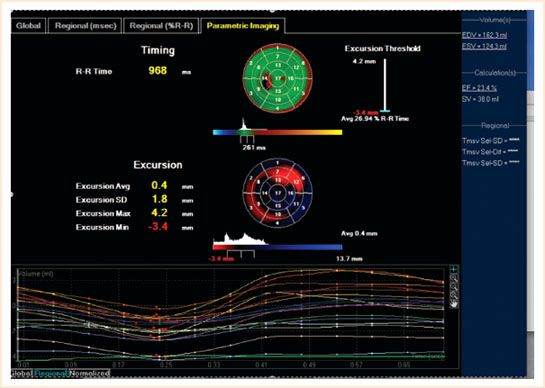
FIGURE 15.1 The “bull’s eye” depicts the standard deviation of the time taken of the individual segments in cross section to contract maximally. This may aid resynchronization therapy.
It is important to score any myocardial segments with RWMAs prior to bypass and evaluate post-CABG for improvement (Table 15.3). Unfortunately, not all RWMAs benefit from coronary revascularization. Most akinetic and dyskinetic regions are the result of myocardial infarction and thus represent nonviable myocardium. In general, hypokinetic segments are viable and may represent active ischemia.
Shortcomings of TEE
RWMAs can be difficult to quantify because the ventricle twists when contracting, causing different myocardial segments to move in and out of the imaging plane during one cardiac cycle. The endocardial and epicardial borders cannot always be completely identified, limiting accurate quantification of wall thickening. Bear in mind that interpreting regional wall motion is an operator-dependent skill, therefore very subjective. RWMAs may also occur in the absence of coronary artery disease, for example, when paced.
NEW RWMA AFTER CARDIOPULMONARY BYPASS
What Does it Signify?
In two classic studies by Roizen and Smith, the occurrence of postoperative myocardial infarction was increased in patients who exhibited new RWMAs during CABG or aortic vascular surgery (24,25). The incidence of postoperative infarction was more strongly associated with new RWMAs than with new electrocardiographic changes. Although TEE may be sensitive in diagnosing ischemia, a new RWMA does not always predict myocardial infarction. In the study of Leung et al. (26), a myocardial infarction was subsequently diagnosed in only one of eight patients in whom a new, persistent RWMA had developed. This apparent discrepancy is consistent with the concept of “myocardial stunning,” in which an acute episode of myocardial ischemia can result in wall motion abnormalities that later resolve without any permanent injury. Alternatively, new RWMAs may be related to loading conditions of the ventricle, electrolyte abnormalities, blood viscosity, level of inotropic support, hypothermia, off-pump CABG stabilizing devices, cardiac pacing, and bundle branch conduction abnormalities. Any apparent dyskinesis due to conduction abnormalities can be differentiated from ischemia by looking for myocardial thickening.
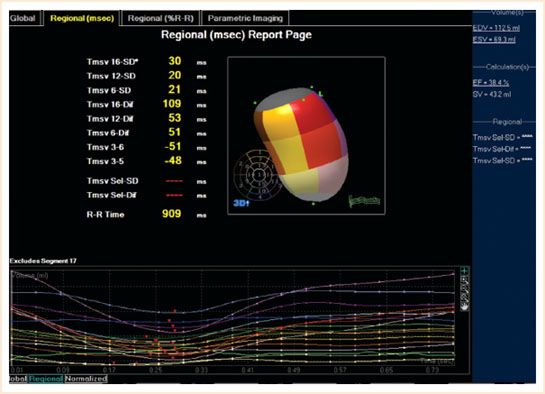
FIGURE 15.2 Complete 3D regional wall motion map. The regional segments’ movements are displayed in milliseconds.
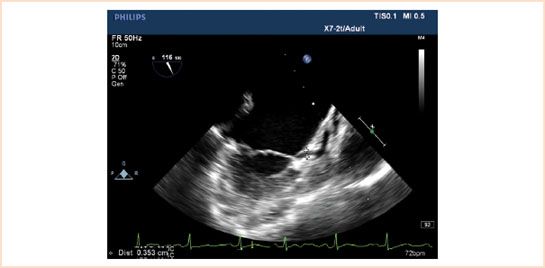
FIGURE 15.3 A 2D demonstration of the circumflex coronary artery visualized in the atrioventricular groove.
TABLE 15.3 Segmental Scores in Increasing Order of Severity

What Should We Do?
The appearance of new RWMAs after bypass is common, but the interpretation is complicated by factors such as infusion of inotropic drugs, inadequate recovery from cardioplegia, conduction abnormalities, and transient ischemia resulting from the distal coronary embolization of air or debris. Although some investigators have suggested that the detection of new RWMAs warrants further surgical intervention, no prospective study is available to support such an aggressive approach. The additional morbidity/mortality associated with the resumption of bypass to place another graft would likely outweigh the potential benefit in most cases. A more progressive approach is presented in Table 15.4.
The surgeon can assess graft patency and flow by visual inspection to confirm the absence of graft kinking or torsion, by stripping the vein graft to confirm refill, by palpation, and by the use of a Doppler flow probe. Decreased flow in an arterial conduit can result from poor distal runoff, a compromised anastomosis, or vasospasm that can be effectively treated with nitroglycerin or a calcium channel antagonist such as nicardipine or nifedipine.
Ventricular septal hypokinesis and dyskinesia is common immediately after separation from CPB and usually recovers within 10 to 15 minutes (Video 15.3). The abnormal conduction progression inherent with RV pacing results in contractile dyssynchrony, which may be interpreted as a septal RWMA. Atrial pacing commonly restores the normal ventricular conduction pathway and contractile synchrony of the ventricular septum. If septal dyskinesia persists then the surgeon should be advised to check the LAD and RCA grafts if present. RWMAs in other areas of the left ventricle need the patency of the graft to be checked sooner. If a RWMA persists or, more commonly, global dysfunction is seen with TEE, then circulatory support should be started either with inotropes or by inserting a circulatory assist device. The level of assist depends on the dysfunction seen. TEE aids placement of the devices and monitoring of recovery.
Differentiation between poor perfusion and stunned myocardium is more difficult. Possible strategies include epicardial scanning and the administration of a contrast agent to determine coronary flow patterns. If the area demonstrates flow of the contrast agent, the RWMA may resolve with time. The absence of contrast agent suggests a technical problem at the anastomosis or distal obstruction in the native coronary. Such information can help guide any possible surgical intervention. The technique of contrast perfusion can also be performed before bypass. If a specific area is likely to be infarcted, as demonstrated by the absence of contrast flow and significant wall thinning, surgical interventions are not likely to be of value. Both these techniques are uncommonly applied in routine operative practice, and their impact remains to be determined.
TABLE 15.4 Strategy for Management of a New RWMA Abnormality after Separation from Cardiopulmonary Bypass

TABLE 15.5 Echocardiographic Findings in Hypotension and Cardiac Dysfunction
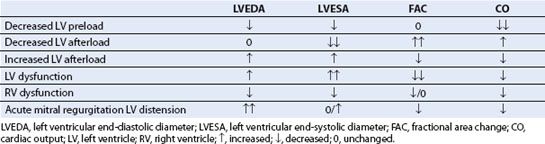
ACUTE CARDIAC DYSFUNCTION: ASSESSMENT AND MANAGEMENT
Cardiac dysfunction may develop at any time during the perioperative period. TEE examination of the heart and great vessels can provide a quick assessment of the primary factors related to hypotension: Preload, afterload, myocardial contractility, valvular function, and integrity of the aorta. TEE can significantly affect the surgical and anesthetic management, especially in high-risk patients or in patients with acute hemodynamic collapse. The reader is reminded that post-CPB cardiac function must be interpreted in the context of the prebypass examination findings and the occurrence of any significant bypass events.
The TEE examination quickly provides data that guides pharmacologic therapy and volume resuscitation. In several studies, TEE significantly modified both surgical and hemodynamic decision-making during the perioperative period (26,27). The echocardiographic findings associated with common causes of hypotension and cardiac dysfunction are presented in Table 15.5 and discussed below:
Hypovolemia
Hypovolemia, a common cause of perioperative hypotension, is often related to the obstruction of venous inflow as a consequence of prebypass cannula placement, volume re-equilibration after separation from bypass, and bleeding. The assessment of LV chamber size by TEE has been shown to be a sensitive measure of LV preload. In the study of Cheung et al. (28) the quantitative analysis of changes in the TG short-axis area could reliably detect even a 2.5% decrease in intravascular volume.
After separation from CPB, the left ventricle is often noncompliant, especially when the LV is hypertrophied. This makes assessment of filling difficult when relying on a pressure reading from a CVP or PAC as higher pressures will be necessary to achieve optimal preload. TEE allows direct visualization of diastolic chamber size (which can be correlated with a pressure reading) and assessment of fluid therapy.
Dynamic Mitral Regurgitation
The development of mitral regurgitation (MR) may be associated with hypotension, increased pulmonary pressures, RV failure, and decreased CO. Excessive volume resuscitation or increased afterload can result in LV distension with incomplete coaptation of the mitral leaflets and a central jet of regurgitation. Alternatively, ischemia or LV dysfunction can lead to papillary muscle dysfunction or LV distension. Significant MR may require mitral valve surgery or hemodynamic management, such as adjusting the systemic vascular resistance, administering inotropic agents, or decreasing the LV preload.
Right Ventricular Dysfunction
RV dysfunction is another common cause of perioperative hypotension. RV dysfunction is associated with RV dilation, tricuspid regurgitation, abnormal septal wall motion, and decreased LV chamber size. Management includes checking for ischemia (though more difficult to assess for the RV), optimizing the pulmonary vascular resistance (PVR), systemic arterial pressure, and contractility. The RV can be affected by pathology of the LV and vice versa. Following myocardial infarction, this ventricular interdependence can affect the shape (e.g., septal shift), size, and function (pressure–volume relationship) of the other ventricle, and is a predictor of outcome. The use of only retrograde cardioplegia can be associated with inadequate RV protection as the balloon on the cannula can obstruct the right cardiac vein preventing cardioplegia reaching the territory supplied by it.
RV systolic dysfunction following CABG is commonly diagnosed by 2D echo images using ME four-chamber, ME RV inflow/outflow, and the TG SAX views. After open-heart surgery any residual intraventricular air is more likely to enter the right coronary because of its anterior position causing temporary RV ischemia and dysfunction. With the open chest, impairment of the free wall and RV distension can be visualized directly. Dysfunction and recovery can also be monitored by TEE while treatment is instituted using rest, administration of inotropic agents that also decrease PVR (e.g., milrinone, dobutamine), and titration of pulmonary vasodilators (nitric oxide, prostaglandin E1, or nitroglycerin) (Videos 15.4, 15.5).
Low Ejection Fraction
Patients who have reduced left ventricular ejection fraction (LVEF) as evaluated prebypass (<40% EF) may need supplemental inotropic or chronotropic support as they are often on β-blockers and the ventricles can appear sluggish on TEE initially upon separation from bypass. This may be short lived and is monitored by TEE and therapy may be discontinued relatively quickly. LVEF <30% prebypass invariably needs inotropic support and TEE is useful in assessing filling and changes in contractility in these patients. An increasingly sluggish LV post-CPB is an indication for insertion of an IABP. If this, in combination with inotropes, does not help then a left ventricular assist device (LVAD) is the next consideration.
Unexpected Diagnosis of Coexisting Problems
Unexpected findings are not uncommon in patients who have undergone a standard workup (7,10) and can be secondary to disease progression or previously undetected findings. Diagnosis of previously undetected pathologies can dramatically alter the surgical plan, for example, insertion of a mitral ring for severe MR, as well as impact the postoperative management and outcome.
Intracardiac Shunts; Patent Foramen Ovale or Atrial Septal Defect
Up to 25% of adult cardiac surgical populations have an intracardiac shunt which can affect the surgical plan and long-term neurologic prognosis due to paradoxical air embolism. If a patent foramen ovale (PFO) is found incidentally during CABG surgery, the decision regarding closure depends on individual surgical preference (29). When an open-chamber procedure is performed in conjunction with CABG surgery or in a patient with a stroke history, an incidental PFO should be closed.
A PFO can be most reliably detected when the interatrial septum is examined with both color flow Doppler and after air contrast injection in the ME four-chamber and bicaval views. The passage of contrast bubbles in the left atrium within five cardiac cycles confirms the diagnosis of a PFO.
Intraoperative detection of a previously unsuspected PFO has relevance to ICU management and emphasizes the need for an echocardiographic report in the medical record and communication with the ICU staff. For example, if atelectasis develops post-CABG surgery resulting in an increase in RV afterload, the increase in right heart pressures can cause the shunt direction to change or exacerbate an existing right-to-left intracardiac shunt, with subsequent worsening hypoxia.
Pleural Effusions
Iatrogenic pleural effusions often develop when the pleura is opened during internal mammary artery harvesting and may be already present in patients with impaired ventricular function. This may have a marked effect on post-CPB ventilation and oxygenation as well as affecting the hemodynamic status of a patient with borderline cardiac function. Ultrasound can easily detect both left- and right-sided pleural effusions. Left pleural effusions can be seen in the ME descending aortic SAX view. Right-sided effusions are seen by turning the probe from the ME four-chamber view to the right (Videos 15.6, 15.7).
Previously Unknown Aortic Valve Pathology
Moderate aortic stenosis may be discovered during intraoperative TEE for CABG surgery. Quantification of AV disease and the decision when to perform a combined CABG and aortic valve replacement (AVR) procedure can be difficult, especially when LV function is compromised and the loading conditions may be altered. Evidence shows that a subsequent AVR after a previous CABG has a higher mortality than a single combined procedure (30). The degree of calcification is important because even in the asymptomatic patient progression of the disease process may be rapid. Progression is quite variable, with a decrease in effective valve area ranging from approximately 0.1 to 0.3 cm2/y. In a primary CABG procedure, it is therefore recommended that AVR should be considered in a patient with even moderate AS.
A patient with coronary artery disease may need insertion of an IABP during ischemic or unstable periods after CPB. If aortic regurgitation is diagnosed intraoperatively, the use of an IABP is contraindicated due to its effect on the LV end-diastolic volume and wall tension.
Coexisting Mitral Valve Regurgitation—Combined CABG and MVR
Patients presenting for CABG often have coexisting functional or ischemic MR (e.g., Carpentier Class I and III) of a mild or moderate degree. The team needs to decide whether or not to surgically address the MV pathology during the coronary operation. The altered loading conditions under anesthesia should be taken into consideration when quantifying the regurgitation. The reduction in ventricular chamber size, systemic vascular resistance, and blood pressure that typically occurs in anesthetized patients can significantly reduce the degree of mitral incompetence compared to the awake state. In order to gain more reliable information we advise to simulate awake loading conditions by increasing both preload and afterload with a vasopressor.
In patients with functional ischemic mild (graded as 1 to 2) mitral regurgitation, the MV is often left untouched. The need for surgical intervention in patients with moderate (>2+) ischemic MR under anesthesia remains a point of debate (Fig. 15.4, Video 15.8). The risk of leaving the MV untouched with the possibility that CABG surgery will successfully decrease the severity of MR by improving LV function and geometry are weighed against the risk of MV surgery with extending aortic cross-clamp times. In patients with ≥3+ ischemic MR, repair or replacement is recommended to improve functional status (31). The final decision whether to proceed with mitral surgery in the setting of ischemic heart disease depends on the surgeon. Importantly, moderate MR secondary to a structural leaflet abnormality (e.g., Carpentier Class II) should be corrected as the disease is progressive and revascularization alone will not improve valve function.
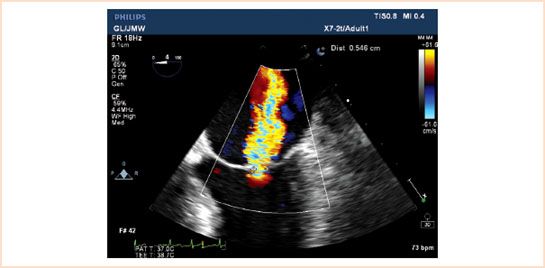
FIGURE 15.4 ME four-chamber view of a patient with a central jet of moderate mitral regurgitation based on a vena contracta of 0.546 cm.
Use of 3D Echo in Coronary Artery Revascularization
RT3D TEE technology is an exciting advance in evaluating ventricular function and although its application during CABG is not routine at present, 3D TEE is emerging as an adjunct to 2D in revascularization procedures to assess cardiac chamber volumes, ischemia, and cardiac output. Compared to 2D imaging, RT3D has lower spatial resolution and also lower temporal resolution but it is more accurate for volumetric measurements. Some have advocated its use for EF and volume assessment in all cases as it removes some of the geometrical assumptions made in 2D, improves accuracy, and removes subjectivity (20,19). Pre- and postvalues of stroke volume are used as an objective measurement to assess improvement post-CABG surgery.
Three-dimensional measurement of LVEF and volumes are made from the ME four- and two-chamber views. The echocardiographer must ensure that the whole of the LV is in view for full-volume acquisition to minimize foreshortening. Ideally ventilation should be stopped while acquiring the image to minimize stitch artifacts for the 5 to 7 beats 3D acquisition. The full-volume image is acceptable when the landmarks of the mitral annulus, septal and lateral points, and apex are easily seen.
There is a high correlation of 3D volume reconstruction by echo with magnetic resonance imaging (MRI) but in general 3D echo slightly underestimates the LV volume due to under tracing of the endocardium. Three dimensional is superior to 2D as surface rendering is not dependent on geometrical assumptions which is important if an aneurysm is present. Changes in EF following revascularization can be quantitatively measured this way (Fig. 15.2).
Segmental wall function can be visualized in both the 3D volume reconstruction and a trace of each of the 17 segments with a different color depicting the time taken to reach minimal volume (i.e., maximum contraction) against time. This should be simultaneous as any delay reveals LV dyssynchrony. The standard deviation of regional ejection times is referred to as the systolic dyssynchrony index (SDI). The report page depicts a bull’s-eye map displaying the 17 segments of contraction as parametric images with color shading for timing differences (Fig. 15.1). All these maps make it easy to assess which segments have delayed contraction and by inference, ischemia and are again imaged postrevascularization to assess for improvement in contractility objectively. LVSDI may be used to predict the response to resynchronization therapy and where to best position the LV lead in the future. The RV is susceptible to ischemia in coronary revascularization. Three-dimensional imaging is a useful adjunct to 2D in assessing function, size, and volume as its crescent shape makes measurement of these parameters difficult in 2D (Video 15.9).
TEE for Specific Surgical Situations
Redo Sternotomy
TEE can be useful in detecting adherence of the right ventricle to the sternum. This is best viewed in the ME RV inflow–outflow plane where restriction of the free wall of the RV can be seen (Video 15.10). Femoral cannulation may be a preferred option in these circumstances.
Off-pump CABG
TEE intraoperative evaluation has aided off-pump coronary artery bypass (OPCAB) graft or minimally invasive direct (MIDCAB) surgery techniques (32). Patient tolerance to the procedure can be monitored in real time with TEE as patients undergoing OPCAB are susceptible to cardiovascular instability especially when the myocardial stabilizer is applied. Application of the stabilizer and displacement of the heart can also cause transient occlusion of the coronary arteries and ischemia which can be detected by TEE. Intraoperative detection of moderate-to-severe MR may indicate that the OPCAB approach is not warranted.
RWMA during OPCABG: Transgastric images are often lost when the heart is displaced to access the right and circumflex coronary arteries. However, evaluating the ME four-chamber, two-chamber, and LAX views, during OPCAB surgery, allows for >14 segments to be evaluated for new RWMAs (33). Placement of a sterile glove filled with saline instead of lap pads posterior to the heart has been shown to improve image acquisition of the TG views (34). If a new RWMA is detected then repositioning of the stabilizer or insertion of an intercoronary perfusion shunt may be beneficial. Persistent abnormalities may predict postoperative problems and graft patency (Video 15.11).
MR during OPCABG: This can be a new development when the heart is displaced and the annulus distorted. It is easily detected by CFD in the ME views. Pre-existing MR may preclude OPCAB surgery as it increases the risk of progressing to severe persistent MR and pulmonary edema (Video 15.12).
IABP insertion during OPCABG: An IABP is used occasionally to support the heart during OPCAB surgery, especially if the LV is poor or the patient has unstable angina. TEE guidance is useful for insertion as the OPCAB approach is commonly selected in patients with severe atheromatous disease of the aorta.
Preload assessment during OPCABG: Diastolic filling can be impaired by the use of a myocardial stabilization suction system and compression of the right atrium can occur when the heart is elevated. The Trendelenburg position helps maintain ventricular filling and TEE can distinguish between hypovolemia and impaired cardiac function.
Conversion to CPB during OPCABG: This decision can be elective or as an emergency response. TEE can anticipate deterioration of ventricular function, persistent RWMAs requiring graft revision, or development of severe MR, before sudden hemodynamic collapse occurs. New information, for example, valvular abnormalities or atrial septal defects, indicating a need for CPB may also be found in these patients.
Graft patency during OPCABG: The patency of the grafts is assessed with TEE by looking for normal function and resolution of RWMAs prior to chest closure. The intraoperative use of myocardial contrast echocardiography may differentiate causes of LV dysfunction immediately after separation from CPB following revascularization by looking at regional flow patterns. Hence, TEE can modify OPCAB cases and may improve morbidity and mortality in these patients (35).
Role of TEE in Vascular Cannulations
Aortic cannula: As part of a complete examination during coronary revascularization, the ascending and descending aorta should be examined for atheromatous plaques or calcification. This will influence surgical decision-making when instrumentation of the aorta is considered, for example, aortic cannula, aortic cross-clamp, or intra-aortic balloon counterpulsation. Detection of severe atheromatous disease of the ascending aorta is a risk factor for poor outcome and modification of surgical technique may be warranted. Changing the aortic cannulation and cross-clamp sites, avoiding an aortic side-clamp by performing all proximal and distal coronary anastomoses during one single ischemic cross-clamp period, and using endoaortic balloon occlusion are all options to reduce embolic consequences. Furthermore, OPCAB surgery or CABG under hypothermic circulatory arrest can also be considered.
The ME ascending aorta LAX and SAX, the ME AV LAX, UE aortic arch SAX and LAX views examine the ascending aorta and arch, and the ME descending aorta SAX and LAX views examine the descending aorta. Due to the anatomical location of the trachea and the left main stem bronchus, which lies between the esophagus and the aortic arch, TEE only visualizes 50% to 80% of the ascending aorta. Atheroma is measured perpendicularly from the outer vessel wall to the intimal surface and graded 1 to 4 depending on the thickness. Grade 5 is a mobile plaque (Fig. 15.5, Videos 15.13, 15.14). Severe atheroma (grade 4 to 5) found in the descending aorta has been correlated with the presence of a similar grade atheroma in the ascending aorta. Detection of severe or mobile plaques in the aortic arch may change the surgical plan converting an on-pump coronary revascularization to OPCAB procedure to avoid the “sand blasting” effect of the aortic inflow cannula disrupting plaque and sending it into the systemic circulation as emboli (35–37). Postcannulation and decannulation assessments are made to check for iatrogenic aortic dissection originating from the aortic cannulation and antegrade cardioplegia sites as well as the proximal vein graft sites. When there is inadequate visualization of the aorta with TEE, epiaortic scanning should be considered.
1. Cardioplegia
Cardioplegia is given either by antegrade and/or retrograde routes. Prior to placement of the antegrade cardioplegia cannula, TEE and epiaortic imaging are used to check for the presence of aortic atheroma in the ascending aorta, arch, and descending aorta. Aortic dissection is a rare complication of antegrade cardioplegia cannulation but may be detected by TEE in the ME ascending aorta LAX view.
TEE is useful to guide and confirm placement of the retrograde cannula in the coronary sinus. The ME four-chamber with slight retroflexion and advancement of the probe provide the most reliable view of the coronary sinus (Fig. 15.6, Video 15.15). Alternatively, the coronary sinus can be visualized in a modified bicaval view with the imaging plane at approximately 130 degrees. The presence of a valve at the entrance to the coronary sinus (Thebesian valve) may impede insertion. It is essential that a persistent left-sided superior vena cava (SVC) be excluded when retrograde cardioplegia is being used as it will render the cardioplegia ineffective. This persistent left-sided SVC is best seen in the ME four-chamber view with the probe slightly withdrawn and is seen next to the left upper pulmonary vein (LUPV) although an enlarged coronary sinus showing positive contrast echo from a left-sided IV is more reliable (Fig. 15.7).
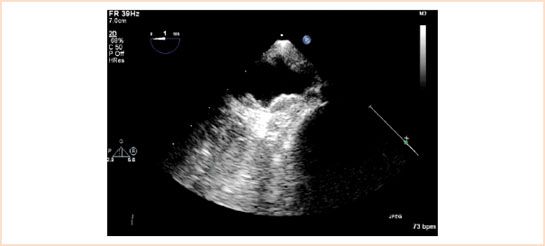
FIGURE 15.5 Grade 4 plaques in the arch of the aorta. This changed the operation from on-pump to off-pump CABG to avoid the “sandblasting” effect. The patient woke up neurologically intact.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree