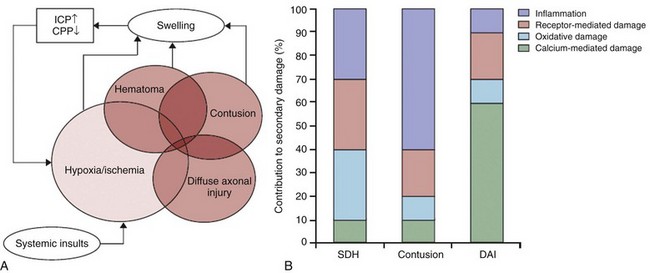
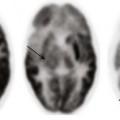
Chapter 39 Birth trauma related to cephalopelvic disproportion, large gestational weight, atypical presentation, and the use of forceps or vacuum extraction is discussed in Chapter 23. Limitations of CT include low contrast in the immature brain, which decreases its sensitivity in detecting edema in infants, and beam hardening artifact, which may partially obscure small extraaxial collection or subtle cortical contusions adjacent to bone, particularly in the posterior fossa and skull base. Although advances in CT scanners have improved their sensitivity in detecting TBI, CT is known to underestimate the degree and extent of traumatic parenchymal injuries when compared with MR imaging (MRI). Nonetheless, acute imaging findings on CT have been proposed as criteria for grading and predicting outcome in persons with TBI (the Marshall Classification and subsequent modifications by Maas et al.).1 CT has been used liberally in children with scalp contusions. Recently, the exposure of children’s brains to excessive radiation has become a concern after Pearce et al.2 demonstrated that children exposed to radiation to the head from CT scans have an increased risk of the development of brain tumors many years later. For patients with a minor head injury (Glasgow Coma Scale [GCS] score of 13 to 15), clinical guidelines are available regarding indications for obtaining a CT scan of the head, such as the New Orleans Criteria, the Canadian CT Head Rule, and guidelines from the Pediatric Emergency Care Applied Research Network, with high sensitivity for detecting injuries that require neurosurgical intervention.3 The American College of Radiology also publishes Appropriateness Criteria for imaging in the setting of head trauma, including a special section for children younger than 2 years in whom clinical assessment and criteria are less reliable than for older children.4 Adaptive statistical iterative reconstruction is a reconstruction technique that reduces image noise and improves low-contrast detectability and image quality. It provides higher diagnostic performance at a lower dose with no loss of image sharpness, noise, and artifacts.5 Because of its more limited availability and longer scanning times, MRI generally is a secondary modality in the evaluation of acute head trauma. MRI usually is performed in the subacute and chronic phases when the findings on CT do not correlate with the patient’s clinical condition, or when patients have unexpected neurologic deterioration or are not responding as expected. MRI offers the advantages of direct multiplanar imaging and greater overall sensitivity and specificity for the detection of TBI. It is particularly useful in the detection of small extraaxial hematomas, nonhemorrhagic intraaxial contusions, edema, brainstem injury, posterior fossa abnormalities, and DAI.6 Contraindications to MRI include incompatible vascular clips, metallic implants, ocular foreign bodies, and most pacemakers. T2* gradient-echo sequence (GRE) uses a pair of bipolar gradient pulses instead of a refocusing 180-degree pulse to enhance magnetic susceptibility caused by magnetic field distortion. Susceptibility-weighted imaging (SWI) is a technique that uses the signal loss from out-of-phase protons with different magnetic susceptibilities. SWI is more sensitive than GRE for detection of blood products such as deoxyhemoglobin, methemoglobin, and hemosiderin.7 Diffusion-weighted imaging (DWI) assesses the diffusion of water protons with use of a bipolar gradient pulse. The normal diffusion of water protons along the gradient reduces the MR signal. Areas of restricted diffusion will retain the MR signal and represent acute cerebral injury in persons with ischemia/hypoxia, trauma, metabolic disorders, and infection. Reduced diffusion is present in lesions with a low nucleus-cytoplasm ratio. DWI is susceptible to the intrinsic T2* signal of the tissue because it is a gradient sequence, known as the T2 shine-through effect. To separate the DWI signal from T2 shine through, the apparent water diffusion coefficient (ADC) is calculated by acquiring images with different gradient duration and amplitude (b-values), thus eliminating the T1 and T2* values.8 Diffusion tensor imaging (DTI) assesses the anisotropy of the brain tissue by evaluating differences in the direction of diffusion of water molecules in normal and abnormal tissues and provides information about the orientation and integrity of the white matter tracts. MR spectroscopy (MRS) assesses the distribution and quantification of naturally occurring molecules within the central nervous system (see Chapter 25). Various techniques are available. The most commonly used technique is the point-resolved spectroscopy sequence. Both short time to echo (TE) and long TE acquisitions can be obtained. All acquisitions provide sensitive, noninvasive analysis of brain metabolites and cellular biochemical changes. Decreased N-acetyl aspartate (NAA) has been reported to correlate with abnormal neuropsychological function tests in persons with TBI.9 Perfusion MRI of the brain can be assessed either through a dynamic T2/T2* acquisition during a bolus injection of intravenous contrast material or with unenhanced techniques such as arterial spin labeling and blood–oxygen level dependent sequences (see Chapters 27 and 28). Perfusion MRI provides the cerebral blood volume, cerebral blood flow, and mean transit time in targeted areas of the brain. Magnetization transfer imaging (MTI) relies on the principle that protons bound in macromolecules of tissues exhibit T1 relaxation coupling with protons in the aqueous phase or water. When an off-resonance saturation pulse is applied, it selectively saturates the protons that are bound in macromolecules. These protons subsequently exchange longitudinal magnetization with free water protons, leading to a reduction in the detected signal intensity. The magnetization transfer ratio (MTR) may provide a quantitative index of the structural integrity of tissue. Animal models of rotational acceleration suggest that quantitative MTI offers increased sensitivity to detection of histopathologically proven damage, such as axonal swelling, compared with conventional MRI. Although the precise mechanism for MTR reduction is incompletely understood for mild head trauma, the extent of the abnormality usually increases as the MTR value decreases. Abnormal MTR also has been found in normal-appearing white matter on MRI and is an apparent predictor of poor outcome.10 Functional MRI has shown changes in regional brain activation in patients with TBI and has been used for assessment of clinical outcome of these patients. Magnetoencephalography (MEG) detects the magnetic waves that are created by the electric current along the axons. The advantage of MEG over electroencephalography is that the magnetic waves are less susceptible to distortion caused by the skull than the electric currents (see Chapter 28). This method of imaging has found that brain dysfunction is present in a significantly greater number of patients with minor head trauma and postconcussive syndrome than is shown by MRI or electroencephalography. This method shows excessive abnormal low-frequency magnetic activity, which provides objective evidence of brain injury in these patients that correlates with the degree of symptomatic recovery. Positron emission tomography (PET) measures the cerebral metabolism of various substrates (see Chapter 25). Fluorodeoxyglucose is the primary substrate used in the measurement of glucose metabolism, which should correspond to neuronal viability. PET can be used in patients with DAI to determine the extent of damage and prognosis. PET also has been helpful in delineating the extent of reversibility of lesions. The major limitation of PET is the inability to distinguish functional abnormalities from structural damage. Based on different mechanisms of trauma and the associated injuries, the sequelae of head trauma can be classified into direct injury related to impact loading forces and indirect injury related to acceleration/deceleration and rotational forces. Direct injury may be subclassified into penetrating and nonpenetrating closed head injury (CHI). Moreover, brain injury in persons with a CHI may result directly from the impact (coup), or it may happen indirectly (contrecoup). The most common sequelae of direct head injury are scalp hematoma, skull fracture, direct brain contusion caused by a fracture and inward deformation of the skull, brain contusion caused by movement against the rough surface of the skull base, indirect (contrecoup) brain contusion diagonal to the site of impact, stretching and laceration of the brain parenchyma, subarachnoid hemorrhage (SAH) as a result of parenchymal injury, and epidural and subdural hematomas caused by direct vascular injury beneath the site of impact. Nonimpact, acceleration/deceleration injuries are the result of forces of translation (linear), acceleration/deceleration, and rotational and angular acceleration causing shear-strain forces on axons, neurons, and blood vessels. These injuries include contusions, DAI, deep gray matter injury, brainstem injuries, intraparenchymal hematomas as a result of vascular injury, and extraaxial hematomas. The axons are most vulnerable, and the blood vessels are the most resistant to injury from acceleration/deceleration forces (Fig. 39-1).11 Based on the GCS, head injuries are classified as mild (GCS score 13 to 15), moderate (GCS score 9 to 12), and severe (GCS score 3 to 8). Most head trauma results in mild injury; the risk of death from minor head injury in childhood approaches 0%. Moderate head injury in children has a similar rate of occurrence as in adults but a lower mortality rate. Severe head injury occurs less frequently in children compared with adults and has a significantly lower mortality rate. The exception is in infants younger than 2 years, who have a higher mortality rate from severe head injuries. This higher mortality rate is partially attributed to the occurrence of NAT in this age group.12 Linear calvarial fractures are more frequent in all age groups, followed by depressed or comminuted calvarial fractures and basilar fractures. Linear fractures usually heal without complication. Complex fractures in which the dura mater is torn may be complicated by herniation of the pia and arachnoid layers, creating a leptomeningeal cyst. The CSF pulsations lead to progressive erosion of skull around the fracture, known as a “growing fracture,” which appears as an angular or linear lytic lesion in the skull with scalloped margins (see Chapter 23). Underlying brain contusion is not uncommon. Depressed fractures are comminuted skull fractures in which broken bones are displaced inward by at least the thickness of the skull. Depressed skull fractures usually result from blunt force trauma to the head and may result in increased intracranial pressure (ICP), epidural hematoma, and parenchymal contusion or laceration. Depressed fractures usually are treated surgically to prevent complications and also for cosmetic reasons. Fractures displacing the dural sinuses often are treated conservatively because of the potential risk of fatal hemorrhage with intervention (e-Fig. 39-2). Depressed fractures are categorized as compound fractures when a laceration of the overlying scalp is present and as penetrating fractures when an underlying dural tear is present, allowing potential communication between the external environment and the brain. Fractures that communicate with the paranasal sinuses, middle ear, or the mastoid air cells within the intracranial compartment are considered compound fractures. Pneumocephalus indicates the presence of a compound fracture (e-Fig. 39-3). The most serious complications of compound skull fractures are CSF leak and infection. Increased risk factors for infection include visible contamination (hair, skin, fat, or bone), a meningeal tear, and delayed treatment for more than 8 hours after the initial injury. A rare type of compound skull fracture is the elevated skull fracture, which occurs when the fracture is elevated outward above the outer table of the skull. e-Figure 39-2 Occipital fracture and cerebellar contusion. e-Figure 39-3 Skull base fracture.
Trauma
Incidence and Etiology
Imaging in Pediatric Head Trauma
Magnetic Resonance Imaging
Other Imaging Modalities
Classification and Mechanisms of Head Injury
Skull Fractures
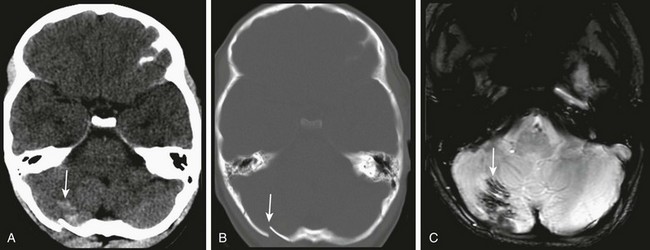
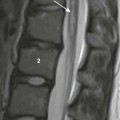
A, Unenhanced computed tomography shows an area of hyperdensity in the right cerebellum representing a hemorrhagic contusion (arrow). B, Bone reconstruction shows a depressed right occipital bone fracture (arrow). C, On the magnetic resonance imaging susceptibility-weighted imaging sequence, the blood products cause low signal susceptibility artifact (arrow). Depressed fractures adjacent to a venous sinus may not be suitable for surgical repair.
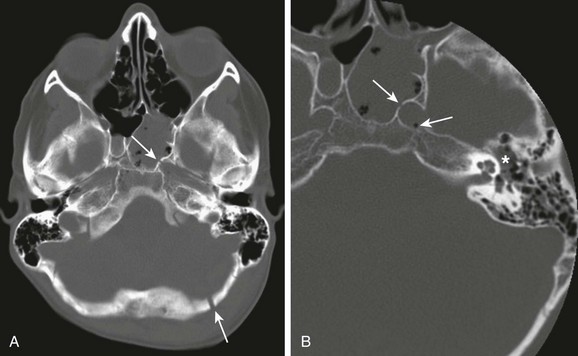
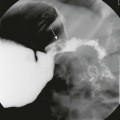
A, A compound fracture through the left occipital bone and petrous apex extending into the sphenoid sinus (arrows). B, High-resolution reconstruction showing involvement of the left carotid canal (arrow) with the presence of a locule of air within the carotid canal (thin arrow) and partial opacification of the middle ear cavity (asterisks).
Trauma