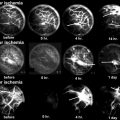
Fig. 14.1
Vascular anatomy of the femoral head. (a) The deep branch of the medial circumflex femoral artery (MCFA) runs towards the intertrochanteric crest between the pectineus muscle medially and the iliopsoas tendon laterally. (b) The MCFA then runs along the inferior border of the obturator externus muscle. (c) After crossing the obturator externus tendon posteriorly, a trochanteric branch is given off which runs between the quadratus femoris muscle and the triceps coxae (gemelli and obturator internus muscles). The deep branch of the MCFA continues cranially and ventrally to the triceps coxae muscle and enters the joint capsule at the level of the superior border of the gemellus superior muscle. (d) At the posterosuperior aspect of the femoral neck, it splits up into four to five retinacular vessels which enter the head (Reprinted with permission Tannast et al. [3])
Multiple anastomoses with the MCFA exist [1]. The most important anastomosis regarding femoral head perfusion is a branch of the inferior gluteal artery, which runs along the piriformis muscle [1, 4]. It could be shown that this vessel has the capacity to compensate for an interruption of the blood supply of the afferent portion of the MCFA [5]. The lateral circumflex femoral artery or the ligamentum teres arteries contribute very little to head perfusion [2]. Usually, the intraosseous blood flow cannot compensate for an intracapsular injury to the deep branch of the MCFA. Therefore, in clinical practice, viability of the femoral head directly depends on the integrity of the MCFA.
14.2.2 Assessment of the Integrity of the Nutrient Vessels of the Femoral Head
Damage to the MCFA can theoretically occur by direct traumatic injury from the accident (i.e., rupture of the vessel), kinking due to fracture and/or joint dislocation, thrombosis, vasospasm, or iatrogenic injury (e.g., forced closed reduction, surgical approach, hardware insertion).
14.2.2.1 Preoperative Modalities for Detection of an Interrupted Blood Supply to the Femoral Head
A reliable, clinically routinely usable, preoperative technique to diagnose an injury of the MCFA with interruption of the blood supply to the femoral head does not exist. Direct visualization of the nutrient vessels to the femoral head is possible by angiography [5, 6]. It can show the interruption of the MCFA but is unable to show the ultimate perfusion of femoral head. Two historic studies [5, 6] tried to correlate the angiographic results in proximal femoral fractures with the occurrence of AVN at most recent follow-up. In hips with preserved blood supply to the femoral head in the angiography, AVN occurred very rarely. In contrast, if the preoperative blood supply to the femoral head was interrupted in the angiography, this did not necessarily end up in an AVN. In these cases, the blood supply can sometimes even be restored by simple closed internal rotation of the hip in femoral neck fractures or after open reduction and internal fixation [6, 7]. This supports the theory of a transient kinking and/or vasospasm of the nutrient vessels. Despite its utility, angiography has not become part of the routine clinical follow-up. Nowadays, CT angiography may be a promising noninvasive alternative to conventional angiography [8]. The MCFA and its anastomoses can be visualized in detail with this technique [8]. However, similar to classic angiography, the terminal intraosseous blood flow to the femoral head is not visible. The usability of this technique in clinical routine has not yet been proven.
There are two imaging modalities that can show the preoperative femoral head vascularity with proximal femoral fractures. The first modality is the dynamic magnetic resonance imaging (MRI), which monitors quantitatively the flow of an intravenously applied contrast agent in the femoral head [9]. The signal intensity of the fractured side before and after application of gadolinium is then compared to the unaffected side. Similarly, bone scintigraphy (as the second modality) uses an intravenously applied radiographic tracer [10, 11]. Unlike dynamic MRI, this method reveals more qualitative results. Despite relative promising results of these two techniques, neither has found its way into clinical routine use.
14.2.2.2 Intraoperative Assessment
One of the most reliable and technically simple methods to assess femoral head perfusion is intraoperative drilling of the femoral head [7]. After reduction of the fracture, two to four 2.0 mm drill holes are made at the base of the femoral head to assess femoral head bleeding. This requires an open approach to the hip and usually takes up to 2 min until reliable bleeding from the femoral head may be observed. If a closed reduction and percutaneous fixation is attempted, the retrograde blood flow through the cannulated screws can provide a relatively reliable assessment of the femoral head perfusion [12]. More sophisticated methods such as laser Doppler flowmetry [13] and intramedullary oxygen tension measurements [14] have been described but basically offer a lower sensitivity and specificity for prediction of AVN in comparison to direct drilling of the femoral head.
14.3 Injury Patterns of the Proximal Femur
The incidence of traumatic AVN of the femoral head depends on the fracture pattern and its proximity to the deep branch of the MCFA (Table 14.1). While the femoral head and neck fractures have the highest incidence of AVN (up to 40 % [18]), the incidence decreases for intertrochanteric fractures (1–5 % [17, 18, 23]), and AVN rarely occurs in femoral shaft fractures [24] (Table 14.1). Femoral head necrosis has also been reported following traumatic dislocations with an incidence ranging up to 26 % [25–27].
Table 14.1
Selected literature reporting on incidence of avascular necrosis of the femoral head following fractures of the proximal femur or traumatic hip dislocation
Study | Fracture pattern | Treatment | Number of hips (patients) | Mean age with range (year) | Incidence of AVN (%) | Results |
|---|---|---|---|---|---|---|
Giannoudis et al. [15] | Head | 23 % nonsurgical | 453 (450) | 39 (6–81) | 12 | Systematic review |
77 % surgical | 3.7 and 2.2 times increased risk for AVN for posterior or trochanteric flip approach compared to anterior approach | |||||
Guo et al. [16] | Head | 18 % nonsurgical | 176 (176) | n.a. | 13 | Systematic review |
82 % surgical | Incidence of AVN of 16 % for posterior, 13 % for trochanteric flip, and 8 % for anterior approach | |||||
Moon and Mehlmann [17] | Head, neck, intertrochanteric | Surgical and nonsurgical | 360 (360) | 10 (1–16) | 21a | Systematic review |
I: 38 | Fracture typea, displacement, age, and treatment (open vs. closed reduction) are risk factors for AVN | |||||
II: 28 | ||||||
III: 18 | ||||||
IV: 5 | ||||||
Yeranosian et al. [18] | Head, neck, intertrochanteric | Surgical and nonsurgical | n.a. (935) | n.a. (1–19) | 23a | Systematic review |
I: 40 | Fracture typea, treatment (open vs. closed reduction), delay in treatment (>24 h), and use of fixation are risk factors for AVN; no association with decompression | |||||
II: 27 | ||||||
III: 20 | ||||||
IV: 5 | ||||||
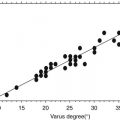
Garden [19] | Neck | 100 % surgical | 406 (n.a.) | n.a. | 23 | Case series |
Incidence of AVN depends on quality of reduction; definition of alignment index; no AVN inside normal range of index; 100 % incidence of AVN with varus (<150°) or valgus (>185°) malreduction | ||||||
Holmberg et al. [20] | Neck | 3 % nonsurgical | 2,251 (n.a.) | 74 (39–99) | 12 | Case series |
97 % surgical | Increased incidence of AVN in hips with operative treatment (12 %) compared to nonoperative treatment (2 %) | |||||
Johnson and Crothers [21] | Neck | 26 % pinning | 153 | 72 (n.a.) | 14 | Case series |
74 % intramedullary nailing | Increased incidence of AVN in hips with intramedullary nailing (16 %) compared to pinning (8 %) | |||||
Strömqvist et al. [22] | Neck | 100 % surgical | n.a. (300) | 78 (18–98) | 7 | Case series |
Increased incidence of AVN in hips with displaced neck fractures (9 %) compared to non-displaced fractures (3 %) | ||||||
Aguado-Maestro et al. [23] | Intertrochanteric | 100 % PFN | n.a. (200) | n.a. | 1 | Case series |
Patient series with intertrochanteric fractures treated with PFN and 1 out of 200 with AVN | ||||||
Orler et al. [24] | Shaft | 100 % intramedullary nailing | 17 (17) | 13 (8–15) | n.a. | Systematic review of case reports with AVN following intramedullary nailing of femoral shaft fractures |
Brav [25] | Dislocation | 93 % closed reduction | 523 (517) | 25 (3–75) | 26 | Case series |
7 % ORIF | Incidence of AVN depends on direction of dislocation, associated fractures, delay in treatment (>12 h) | |||||
Epstein [26] | Dislocation | Closed reduction and ORIF | 242 (242) | n.a. | 18 | Case series |
Incidence of AVN depends on associated fractures | ||||||
Hougaard and Thomsen [27] | Dislocation | Closed reduction and ORIF | 100 (98) | 39 (16–89) | 13 | Case series |
Incidence of AVN depends on delay of treatment (>6 h) |
Fractures of the proximal femur are divided into head, neck, intertrochanteric, and femoral shaft fractures. For each fracture pattern, the etiology of AVN, the anatomical relation to the MCFA, and the incidence of AVN reported in literature are summarized.
14.3.1 Femoral Head Fractures
Among the different fracture types of the proximal femur, femoral head fractures have the highest incidence for AVN ranging up to 40 % (Table 14.1) [18]. Femoral head fractures typically are related to direct mechanical damage to the intraosseous blood flow from the retinacular vessels (Fig. 14.2). The vascularity of the femoral head fragment can only be reestablished by diffusion from the viable femoral head portion. Based on two systematic reviews [15, 16], an increased risk for AVN has not been found for a specific subtype of femoral head fracture or concomitant traumatic dislocation. However, in both reviews [15, 16], the risk of AVN was associated with the type of approach used for surgical treatment (Table 14.2). The highest incidence of 16 % was found for the posterior approach, followed by the trochanteric flip approach with 13 % and the anterior approach with 8 % [15, 16]. These results suggest that an iatrogenic lesion to the nutrient vessels of the femoral head may play an important role. The posterior approach to the hip can potentially be dangerous for the blood supply to the femoral head if the topographical course of the MCFA is not fully understood and respected (Fig. 14.1). If a trochanteric osteotomy is conducted, the osteotomy should exit just anterior to the most posterior insertion of the gluteus medius muscle in order to protect the deep branch of the MCFA [34].
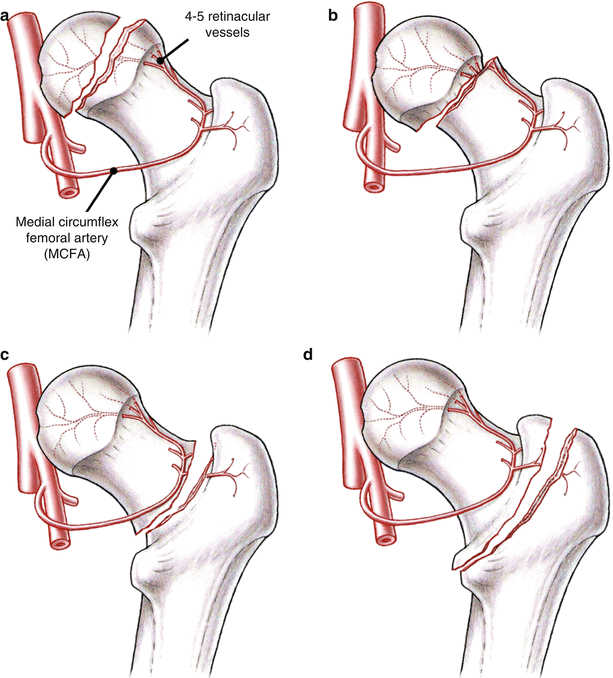

Fig. 14.2
(a) Femoral head fractures have the highest incidence of avascular necrosis (AVN) of the femoral head ranging up to 40 % [18] and typically are related to direct mechanical damage to the retinacular vessels. (b) In medial neck fractures, direct mechanical trauma to the nutrient vessel results in increased risk for AVN compared to (c) lateral neck fractures which less likely interfere with the MCFA. (d) AVN rarely occurs in hips with intertrochanteric fractures because these fractures usually do not interfere with the nutrient vessel
Table 14.2
Fracture pattern with corresponding risk factors and incidence of avascular necrosis of the femoral head
Fracture pattern | Risk factor for AVN of femoral head | Description/incidence of AVN |
|---|---|---|
Head | Posterior approach with incidence of 16 %, trochanteric flip with 13 %, and anterior approach with 8 % | |
Neck | Medial neck fractures with incidence of 28 % compared to lateral fractures with 18 % | |
Adults: dislocated fractures with incidence of 9 % compared to non-dislocated fractures with 3 % | ||
Children: dislocated fractures with incidence of 25 % compared to non-dislocated with 9 % | ||
Quality of reduction [19] | Anatomical reduction with incidence of 7 % compared to severe valgus/varus with 100 % | |
Adults: increased incidence of 16 vs. 0 % for a delay of >12 h | ||
Children: four times increased risk for a delay of >24 h | ||
Adults: incidence of 12 % for surgical treatment vs. 2 % for nonsurgical treatment, 16 % for open reduction vs. 8 % for closed reduction
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|