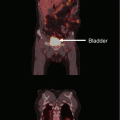
Fig. 1
Intensity-modulated radiation therapy for an eye metastasis. Dose delivered was 25 Gy in five fractions
2.2 Primary Ocular Tumors and Benign Ocular and Orbital Conditions
Primary tumors, specifically melanomas, lymphomas, vascular lesions, and retinoblastomas, present for treatment less frequently than do metastatic ocular lesions. Doses for primary ocular tumors vary widely. Ocular melanomas receive very high uniform doses (50–70 Gy in four or five fractions) most commonly with proton radiation, and similar or even higher nonhomogeneous doses are delivered with episcleral radionuclide plaque therapy (Courdi et al. 2010; Dendale et al. 2006; Gragoudas et al. 2002; Egger et al. 2001; The American Brachytherapy Society – Ophthalmic Oncology Task Force 2014; Badiyan et al. 2014; Barker et al. 2014; Sagoo et al. 2011; Semenova and Finger 2013). Intermediate doses (36–46 Gy) are given for retinoblastoma (Mouw et al. 2014; Merchant et al. 2002; Pradhan et al. 1997) and sometimes even lower doses for ocular lymphomas (Harada et al. 2014; Munch-Petersen et al. 2015; Fasola et al. 2013). Benign ocular conditions, such as macular degeneration; vascular tumors, such as choroidal hemangiomas; and benign orbital conditions, such as Graves’ ophthalmopathy and orbital pseudotumor, may also be treated with radiation (Jackson et al. 2015; Chan et al. 2010; Petersen et al. 1990; Lanciano et al. 1990). In the former condition, only the immediate macular area is targeted, while in the latter two conditions, the entire globe posterior to the ora is irradiated despite the fact that the pathological condition being targeted is in the extraocular muscles or the orbit, rather than within the globe itself. Relatively low doses (10–20 Gy) are given to patients with benign conditions (Barak et al. 2005; Chen et al. 2014; Chan et al. 2010; Petersen et al. 1990; Mourits et al. 2000; Cardosa et al. 2012). More recent investigation with stereotactic radiotherapy doses of 16 or 24 Gy for age-related macular degeneration find early efficacy in data reported at 2 years, but long-term data will be required to assess late toxicity and may impact on eligibility of potential future reirradiation (Jackson et al. 2015). Similar efficacy has been reported with the use of proton therapy of 16 or 24 Gy(RBE) divided in two fractions (Chen et al. 2014).
3 Reirradiation
3.1 Reirradiation After Low Doses Given for Benign Diseases
The low doses given for macular degeneration can be repeated with relatively little risk, although the efficacy of intraocular anti-angiogenic drugs has largely supplanted the use of radiotherapy for macular degeneration. A recent update of indications for treatment of benign disease concluded that macular degeneration is no longer an indication for radiotherapy (Van Houtte et al. 2005). Relatively low doses (20 Gy in 10 fractions) are given to the eye with fractionated external beam photon therapy for Graves’ ophthalmopathy and orbital pseudotumor. That dose can typically be repeated with a low risk of significant morbidity, other than possibly cataract formation if the lens was not adequately shielded during either the first or the second course of treatment. However, there is little if any data available in the literature regarding indications for and either the efficacy or the risk of retreating patients previously irradiated for these conditions.
3.2 Reirradiation After Intermediate Doses Given for Retinoblastomas, Lymphomas, and Metastatic Carcinomas
Patients with these tumors usually are treated with fractionated external beam supervoltage photon therapy to doses of 30–46 Gy ranging at 1.8–3.0 Gy per fraction. The target may include the posterior globe only in patients with posterior retinoblastomas or metastatic lesions. The entire globe posterior to the ora serrata is treated in retinoblastoma patients with more anteriorly located tumors and in patients with diffuse lymphomatous or carcinomatous involvement of the retina or the vitreous. When the aim is to treat only the retina posterior to the equator, appropriate planning and treatment techniques can limit the lens dose to ≤4 Gy, a subthreshold level for cataractogenesis (Parsons et al. 1983). When the target volume extends anteriorly to the ora serrata, the probability of radiation-induced cataract is increased. The threshold dose for radiation retinopathy or optic neuritis is >20 Gy. However, symptomatic retinopathy or optic neuropathy is seen relatively infrequently after doses of the order of 40–46 Gy. Reirradiation after an initial course would likely result in cataract formation, unless the dose to the lens could be kept to a very low level, perhaps by using focused treatment techniques such as proton therapy, episcleral radionuclide plaque therapy, or stereotactic radiotherapy. The use of such focused techniques could also limit the volume of retina and possibly the dose to the macula or the optic nerve. However, systemic treatment, with chemotherapy in patients with ocular lymphomas, retinoblastomas, or metastatic carcinomas may preclude the need to consider reirradiation. Local treatments, using cryotherapy, laser photocoagulation, or radionuclide episcleral plaque therapy, are effective for focally recurrent disease in retinoblastoma patients, and the indication for external beam reirradiation of such patients arises rarely, if at all.
3.3 Reirradiation After High Doses Given for Ocular Melanomas
Ocular melanomas receive very high uniform doses (50–70 Gy in 4 or 5 fractions) with proton beam radiation. Similar or even higher nonhomogeneous doses are also delivered to such tumors with episcleral radionuclide plaque therapy, delivered over 4–8 days, depending upon the intensity of the radiation sources in the plaque at the time of placement. These focused techniques deliver the prescribed dose of radiation to only that portion of the eye containing the tumor. An example of a patient treated twice with proton therapy for a uveal melanoma that subsequently failed marginally is demonstrated in Fig. 2. Non-involved eye structures located away from the tumor receive little or no dose, although small portions of the eye immediately adjacent to the tumor and eye structures through which the beam passes en route to the tumor may also receive the prescribed dose.


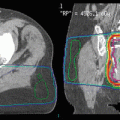
Fig. 2
Uveal melanoma recurrence. An 81-year-old man with a primary left uveal melanoma (a) who was treated with proton therapy to 50 Gy(RBE) in five fractions (b). He recurred along the lateral margin to the primary tumor 14 months later (c) at which time he was reirradiated to 70 Gy(RBE) in five fractions (d)
Reirradiation of new tumors arising away from the initially treated volume and, in certain cases, regrowing immediately adjacent to or even within the previously irradiated volume can be carried out with proper patient selection. Marucci et al. (2006) evaluated the outcomes of a second course of proton beam radiation therapy (PBRT) in 31 patients with recurrent uveal melanoma. Mean interval between the first and the second PBRT course was 50.2 months (range, 8–165 months). Most patients (87 %) received 70 Gy(RBE) (gray-relative biological effectiveness; biological correction to be equivalent to photon doses) for both the initial and the second treatment course. Visual acuity was 20/200 or better in 30 patients initially and in 22 patients at the time of the second treatment. The mean follow-up time after the second treatment was 50 months (range, 6–164 months). When last seen, 20 tumors had either regressed or showed no evident tumor progression. Nine eyes (29 %) were enucleated, five for local recurrence and four because of intractable pain. The 5-year eye retention rate was 55 % (95 % confidence interval, 25.2–77.4). Six of the 22 patients who retained their eye (27 %) had useful vision (20/200 or better). This small retrospective study demonstrated that a second course of PBRT for recurrent uveal melanoma to total doses between 118 and 140 Gy(RBE) was associated with a moderately good probability of local control and a relatively low enucleation rate. Although most patients lost their vision, the majority were able to retain the reirradiated eye.
In a subsequent study, survival in patients with recurrent melanomas was retrospectively compared between those treated by enucleation and those who were reirradiated (Marucci et al. 2011). Patients selected for reirradiation were slightly older than those treated with enucleation (56 vs. 61 years, respectively). Both initial and recurrent tumors were larger in patients treated with enucleation than in those who were reirradiated. Tumor location and the presence or absence of ciliary body involvement did not differ significantly between the groups. The median follow-up after enucleation and after retreatment was longer in the enucleated patients (79 and 59 months, respectively). Median survival duration in the enucleated and reirradiated groups was 42 and 90 months, respectively. The median time free of metastases was 38 months in enucleated patients and 97 months in reirradiated patients. At 5 years after surgery or reirradiation, the probability of overall survival was 36 % and 63 %, respectively (p = 0.040, log-rank test). The probability of freedom from metastases was 31 % and 66 %, respectively (p = 0.028, log-rank test). These differences persisted after adjustment for largest tumor diameter and volume at the time of reirradiation or enucleation. Results of this analysis suggested that survival in reirradiated patients was not compromised by their receiving a second course of proton beam therapy, relative to that of those treated with enucleation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree