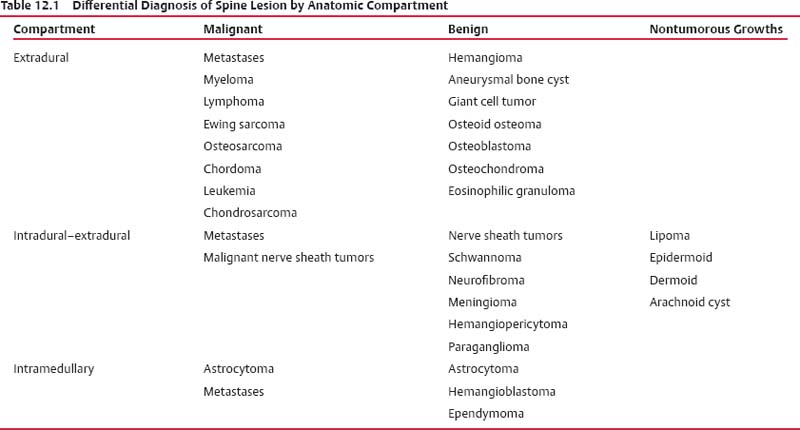
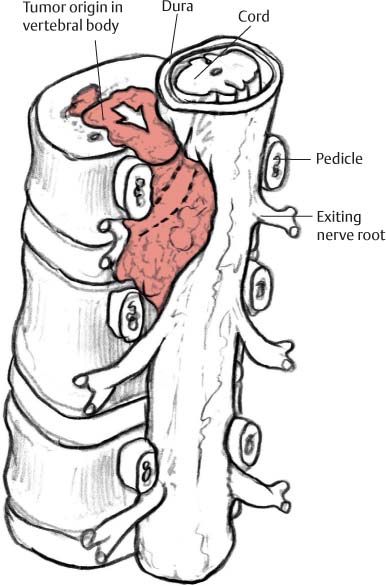
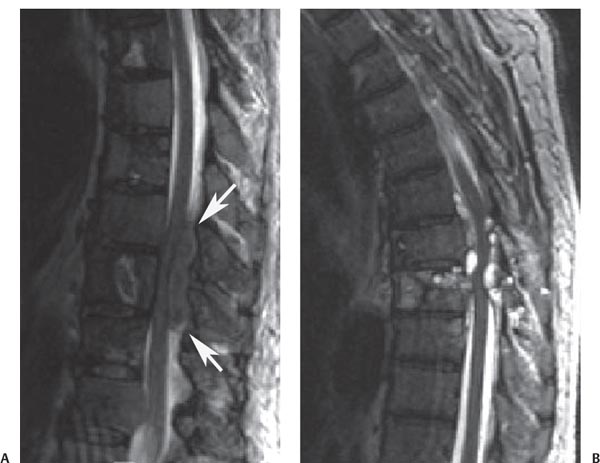
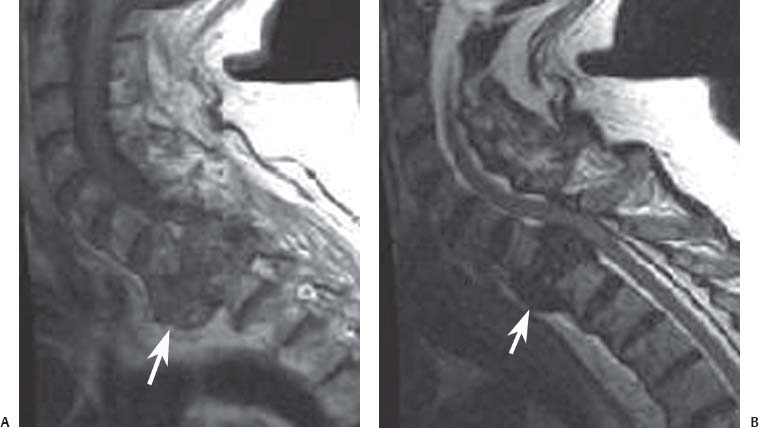
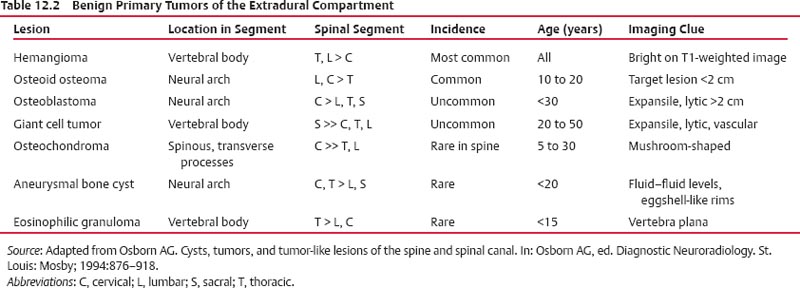
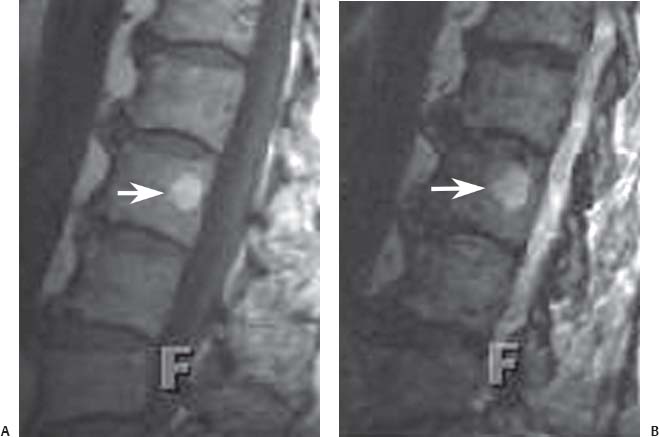
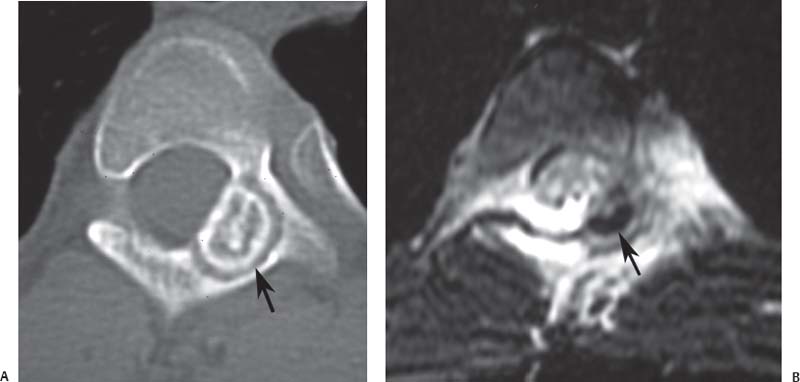
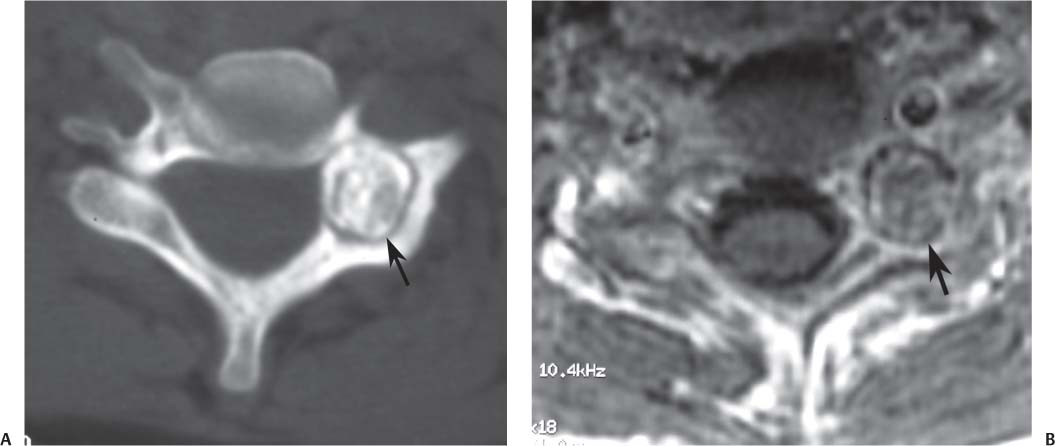
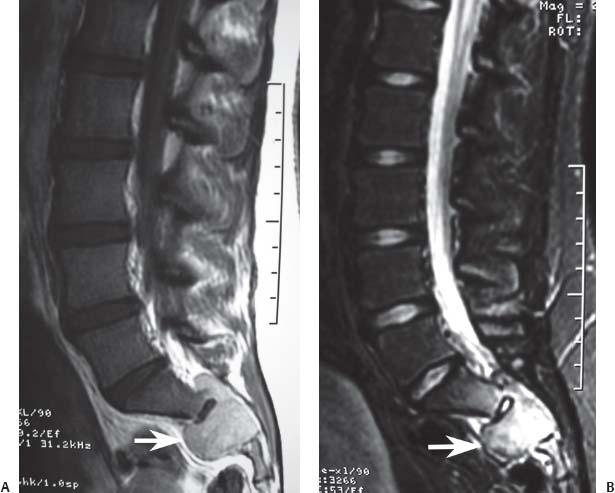
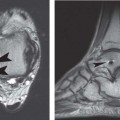
12 Tumors of the Spine Spine tumors are traditionally classified by anatomic location into three compartments:1–6 • Extradural • Intradural–extramedullary • Intramedullary The extradural compartment consists of all structures outside the dura, including the osseous structures, the paravertebral region (including the paraspinal musculature), and the epidural space. Although orthopaedic surgeons most commonly manage tumors of the extradural compartment, they must have an understanding of the other two compartments to provide comprehensive patient care and to communicate effectively with neurosurgical colleagues. Spine tumors also are classified by type of origin as primary or metastatic. Although a large number of primary lesions may occur in the spinal cord, nerve roots, dura, and osseous spine, most lesions within the spine are metastatic tumors.7 Such lesions occur primarily in the extradural compartment, especially in the osseous structures. As systemic therapies for metastatic disease have improved and the life expectancy of such patients has increased, the incidence of metastatic spread to the spine has also increased. Up to 40% of patients with cancer develop visceral or osseous metastases, and the spinal column is the most common site of osseous metastases.8 Prostate, lung, and breast cancer account for most of such lesions.1,3 Metastases can occur in any compartment of the spinal column, but the vertebral body is the site most commonly affected (approximately 85%),9 followed by the paravertebral region, epidural space, and intradural compartment. In addition, although all segments of the spine can be affected, such lesions occur most often in the thoracic spine (approximately 70%), followed by the lumbar spine (20%) and then the cervical spine and sacrum (10%).9 Epidemiologic data suggest that most patients with suspected spine tumors are eventually shown to have meta-static (rather than primary) disease in the vertebral body (rather than in other locations) and that metastatic and primary lesions (benign and malignant) can occur within any segment and compartment of the spine and in men or women of almost any age. Although most lesions have particular identifying characteristics, it is still imperative that, to arrive efficiently and effectively at the correct diagnosis of a spine tumor, the following steps are followed rigorously when reviewing imaging studies4,6: 1. The compartment location of the lesion in the spinal column (extradural, intradural–extramedullary, or intramedullary) must be identified. Such localization may provide a narrowed differential diagnosis. 2. The clinician generates a preimaging differential diagnosis based on patient demographics and clinical characteristics, such as patient age, sex, medical history, and neurologic signs and symptoms (Table 12.1). Such a meticulous evaluation not only guides the type and location of such imaging, but also provides substantial insight as to the true underlying pathology. In this way, imaging serves to corroborate or refute, rather than merely suggest, the previously hypothesized differential diagnosis. 3. The patient’s demographic information and clinical presentation are used to narrow the differential diagnosis. For instance, tumors that present in pediatric patients often are extremely rare in adults and vice versa. In addition, in patients with a history of cancer, neck pain, or back pain should be assumed to be a symptomatic spinal metastasis until proven otherwise. 4. Various imaging modalities (conventional radiographs, CT, or MRI) can be used to narrow the differential to a working diagnosis. Although these steps often may lead the clinician to the correct diagnosis, it should be noted that the working diagnosis based on current imaging techniques is not always accurate. For this reason, image-guided or open biopsy often has a role in obtaining a definitive diagnosis before the initiation of a proposed treatment plan (surgery, radiation therapy, chemotherapy, etc.). MRI is the preferred imaging modality for evaluating most disorders of the spine, including spine tumors.10,11 MRI is more sensitive than conventional radiographs, CT, or bone scans in detecting primary malignant bone tumors and metastatic lesions in the spine.12,13 This increased sensitivity results from the fact that MRI allows for superior resolution of soft-tissue structures, such as the intervertebral discs, spinal cord, nerve roots, meninges, and paraspinal musculature. In addition, MRI provides clarity at the osseous–soft-tissue interface, yielding precise anatomic detail of osseous compression or invasion of neural and paraspinal structures. Despite this excellent anatomic and soft-tissue depiction, however, MRI is limited in its detection of calcifications and small osseous fragments3 and is subject to metal-induced artifact when used in the instrumented spine (usually with stainless steel or titanium), which may prohibit accurate assessment of neural compression and necessitate a fluoroscopic or CT myelogram for optimal evaluation. CT also provides superior osseous detail and permits resolution of calcification in areas within or around soft-tissue structures. Therefore, the ideal method of evaluating a patient with a suspected or known spinal mass involves a combination of conventional radiographs and/or CT imaging with MRI. This chapter describes image interpretation techniques that permit identification of the compartment and the MRI appearance of the most common tumors in each location, facilitating the systematic and efficient creation of a differential diagnosis (Table 12.1) for any spine tumor evaluated with MRI. MRI protocols vary widely among institutions and in relation to the spinal region involved. Despite such variation, the MRI protocol for any patient suspected of having a spine tumor should include T1-weighted and T2-weighted images and gadolinium-enhanced studies in the axial and sagittal planes.4 Gadolinium enhancement often provides improved anatomic detail of spinal tumors and may supply signature clues to the underlying pathology. Because of the high signal intensity of fat within an adult’s marrow on T1-weighted images, fat-suppression techniques are useful in evaluating osseous lesions that enhance with contrast.4 Similarly, enhancing lesions in the epidural space are better seen with fat suppression, particularly in the lumbar spine in which the epidural space is composed primarily of fat. Gradient-echo sequences are not as useful in assessing spine tumors unless hemorrhage is suspected. On the other hand, diffusion-weighted imaging, which often reveals restricted diffusion in a vertebral body with a tumor, may be helpful in distinguishing benign and pathologic compression fractures.14 Consultation with a neuroradiologist generally is advisable for the selection of the ideal imaging protocol. Extradural masses typically arise from the osseous spine, intervertebral discs, and adjacent soft tissues. With MRI, the hallmark of such lesions is focal displacement of the thecal sac away from the mass with an obliterated subarachnoid space and compressed spinal cord (Fig. 12.1). The dura often appears to be draped over the mass. The most common malignant extradural masses are metastases (Fig. 12.2), followed by primary malignant tumors of the spine.5 The most common benign extradural masses are degenerative and traumatic lesions, such as disc herniations, osteophytes, and fractures, followed by primary benign tumors of the spine.5 In this chapter, such lesions pertain to neoplastic lesions of the spine, both malignant and benign. Fig. 12.1 Artist’s sketch (posterior oblique view) depicting the characteristics of an extradural mass. The spinal cord and dura are displaced. The mass typically extends from the vertebral body (arrow) and into the extradural space. The spinal column is the most common site of osseous metastases, and the most common extradural malignant spine tumors in adults are metastatic lesions.5 Autopsy studies reveal vertebral metastases in up to 40% of patients with systemic cancer, with 5% of adults presenting with epidural spinal cord compression.7,8,15,16 Most spine metastases in adults arise from lung, breast, and prostate cancer, followed less frequently by lymphoma, melanoma, renal cancer, sarcoma, and multiple myeloma.17 Approximately 5% of children with solid malignant tumors develop spinal metastases with cord compression.18 In such cases, spine metastases most often are caused by Ewing sarcoma and neuroblastoma, followed by osteogenic sarcoma, rhabdomyosarcoma, Hodgkin disease, soft-tissue sarcoma, and germ cell tumors.18 In adults, the initial site of metastatic tumor growth in the spine typically is the posterior vertebral body, followed by the epidural space and pedicle.19 Conversely, metastatic tumors in children typically invade the spinal canal via the neural foramina, causing circumferential cord compression.18 Spinal metastases in the adult can occur at any level, but they usually involve the lower thoracic and lumbar spine secondary to a higher proportion of red bone marrow in those locations.12,19 Fig. 12.2 Sagittal T2-weighted images of metastatic lesions, revealing compression and distortion of the underlying dura and cord. (A) Prostate metastasis in the thoracolumbar spine (between arrows). (Other images confirmed that the lesion arose from the posterior elements.) (B) Non–small-cell lung cancer metastasis to the osseous thoracic spine with extradural extension. Fig. 12.3 Sagittal T1-weighted (A) and T2-weighted (B) images of a cervicothoracic metastasis from thyroid carcinoma. The expansile lesion is centered in the collapsed vertebral body (arrow on each) and compresses the spinal cord. MRI is the method of choice for imaging metastatic spine disease because of its unparalleled ability to delineate epidural and paraspinous soft-tissue involvement. Imaging patterns of such lesions can reveal focal lytic, focal blastic/sclerotic, diffuse homogeneous, and diffuse inhomogeneous lesions.5 The most common pattern seen is multifocal lytic lesions that are hypointense on T1-weighted images and hypo- to hyperin-tense on T2-weighted images (Fig. 12.3). Sclerotic lesions tend to be hypointense on T1-weighted images and T2-weighted images. diffuse homogeneous and inhomogeneous lesions are hypointense on T1-weighted images and hyperintense on T2-weighted images, although the signal pattern can be variable and depends on the degree of fatty marrow. Contrast enhancement of such lesions also is extremely variable. MRI also may be useful for distinguishing benign, osteoporotic fractures from pathologic, tumor-related fractures. Benign fractures typically have marrow signal intensity identical to that of neighboring normal vertebral bodies. Pathologic fractures are more hypointense on T1-weighted images and more hyperintense on T2-weighted images than are normal vertebral bodies.20 Additionally, diffusion-weighted images, combined with apparent diffusion coefficient mapping, often shows more extensive restricted diffusion with pathologic fractures than with osteoporotic fractures.4 The identification of epidural or paraspinal soft-tissue involvement also is helpful for confirming a pathologic etiology. Benign primary tumors of the extradural compartment of the spine have characteristic MRI findings and patient demographics (Table 12.2) that help differentiate them from malignant lesions. Vertebral hemangioma is the most common spinal axis tumor.21 This benign vascular tumor of the vertebral body, often discovered incidentally on imaging, can be associated with vertebral body collapse and epidural extension with spinal cord compression; on rare occasions, it may exhibit aggressive growth.22 MRI sequences of the typical (fatty) hemangioma show lesions that are hyperintense on T1-weighted and T2-weighted images, with robust contrast enhancement23 (Fig. 12.4). Vertebral hemangiomas are one of the very few spinal tumors that show increased signal intensity on T1-weighted images and T2-weighted images. Occasionally, such lesions are more vascular and may appear isointense or hypointense on T1-weighted images, making them difficult to distinguish from metastases. Although CT images show the typical “polka dot” appearance on axial images and the typical “corduroy” or “jailhouse striation” pattern on sagittal images, secondary to the thickened trabeculae, MRI is the best modality for characterizing the epidural extent and cord compromise of aggressive lesions. Although such lesions primarily involve the vertebral body, 10% to 15% have concomitant involvement of the posterior elements.21 Multiple lesions are seen in 25% to 30% of patients.24 This benign, osteoid-producing tumor, usually <1.5 cm in size, is often surrounded by a ring of sclerotic bone. Almost all of these lesions involve the neural arch, and most occur within the lumbar spine, followed by the cervical, thoracic, and sacral regions. Bone scintigraphy and CT are generally more helpful for detecting and characterizing these lesions than is MRI. The nidus is hypo- or isointense on T1-weighted images and varies from hypo- to hyperintense on T2-weighted images, often with surrounding hyperintensity that likely is related to a local inflammatory response (Fig. 12.5).25 The rapid enhancement pattern, typically located within the nidus, is best seen on dynamic sequences (e.g., serial postcontrast images). The surrounding reactive zone enhances more slowly with such imaging. Of note, up to 70% of patients may present with scoliosis, related to muscle spasm, with concavity on the side of the tumor.26 Fig. 12.4 Thoracolumbar vertebral hemangioma. This asymptomatic lesion (arrow on each) is centered in the vertebral body and appears hyperintense on T1-weighted (A) and T2-weighted (B) sagittal images. Fig. 12.5 Left-side thoracic osteoid osteoma. (A) An axial CT image shows characteristic hyperdense center with sclerotic rim (arrow). (B) An axial T2-weighted image reveals a hypointense center (arrow) corresponding to sclerotic bone surrounded by a region of high signal intensity corresponding to reactive edema. Osteoblastomas, also known as giant osteoid osteomas, are similar histologically to osteoid osteomas but are differentiated from them largely by size (>1.5 cm). Clinical symptoms also can help to distinguish these lesions: osteoblastomas cause a dull pain as opposed to the intense night pain caused by osteoid osteomas. Occasionally, osteoblastomas possess atypical features and behave aggressively. Like osteoid osteomas, these lesions originate in the neural arch but exhibit greater mass expansion.5 Thus, they may be centered in the pedicle, lamina, transverse or spinous process, articular pillar, or pars interarticularis, with extension into the vertebral body. The lesion is hypo- or isointense on T1-weighted images and iso- or hyperintense on T2-weighted images with extensive peritumoral edema (flare phenomenon); fluidfluid levels are often present within the lesion (Fig. 12.6). Contrast enhancement is variable.27 Unlike osteoid osteomas, scoliosis may occur convex toward the side of the tumor. Fig. 12.6 Left-side cervical osteoblastoma. (A) An axial CT shows a lesion (arrow) similar to (but larger than) the osteoid osteoma shown in Fig. 12.4A. (B) An axial postgadolinium T1-weighted image shows the lesion (arrow). Fig. 12.7 Sacral giant cell tumor. This expansile lesion shows intermediate signal intensity on a sagittal T1-weighted image (arrow) (A) and high signal intensity on a sagittal fat-suppressed T2-weighted image (arrow) (B). Giant cell tumors are locally aggressive, lytic tumors in the vertebral body and sacrum and are named for the osteoclast-like giant cells that are present on histology. Hemorrhage is common secondary to the hypervascular stroma of these lesions. MRI shows expansile lesions with hypo- to isointense signal on T1-weighted images and iso- to hyperintense signal on T2-weighted images (Fig. 12.7). Contrast enhancement often is heterogeneous, commonly surrounding areas of necrosis, blood, blood degradation products, and/or cystic cavities with fluid–fluid levels.28 Such lesions can undergo sarcomatous transformation (10% of cases) to become malignant giant cell tumors,29 and thus patients with these tumors must be monitored with MRI or CT because of the risk of recurrence. It is important to note that the differential diagnosis of midline tumors in the sacrum includes giant cell tumor, chordoma, aneurysmal bone cyst, plasmacytoma, and metastases in adults, and sacrococcygeal teratoma in children.9 Osteochondroma, also known as osteocartilaginous exostosis, consists of cartilage-covered osseous protuberances with a medullary cavity that is contiguous with the parent bone. These lesions grossly appear to be cauliflower- or mushroom-shaped lesions with cartilaginous caps. Only 5% of these growths occur within the spine (compared with 85% in long-bone metaphyses), but when they do, they are located mostly in the cervical spine, particularly at C2.9 These lesions most commonly arise from the spinous and transverse processes, but they also may arise from the vertebral body. On T1-weighted and T2-weighted images, they appear as central hyperintense lesions surrounded by hypointense calcified cortex. The cartilaginous cap is hypo- to isointense on T1-weighted images and iso- to hyperintense on T2-weighted images and exhibits peripheral enhancement of cartilage (Fig. 12.8).30 MRI is the preferred imaging modality for measuring the cartilaginous cap and determining the status of regional neural and musculoskeletal tissue. Thickening of the cap (>1 cm) should raise concern for malignant transformation to chondrosarcoma. CT may be used for evaluating the osseous structure of the lesion, confirming the contiguity of the medullary cavity with the parent bone, and evaluating for fractures. These lesions are expansile benign neoplasms containing thin-walled cavities filled with blood and blood products that occur most commonly in patients less than 20 years old. A substantial proportion of aneurysmal bone cysts are associated with preexisting osseous lesions, such as an osteoblastoma, giant cell tumor, chondroblastoma, nonossifying fibroma, or fibrous dysplasia.31 They arise in the neural arch, but most extend into the vertebral body. The classic presentation is a balloon-like expansile remodeling of bone, leaving a thinned “eggshell” cortex. On T1-weighted and T2-weighted images, the lesions appear as lobulated neural arch masses, commonly with extension into the vertebral body, epidural space, and adjacent vertebral bodies and ribs.32
 Specialized Pulse Sequences and Imaging Protocols
Specialized Pulse Sequences and Imaging Protocols
 Extradural Tumors
Extradural Tumors
Metastatic Disease
Primary Benign Tumors
Vertebral Hemangioma
Osteoid Osteoma
Osteoblastoma
Giant Cell Tumor
Osteochondroma
Aneurysmal Bone Cyst
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree