, Fabio Triulzi1 and Andrea Righini2
(1)
Department of Neuroradiology, Fondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico, Milan, Italy
(2)
Pediatric Radiology and Neuroradiology Department, Children’s Hospital V. Buzzi, Milan, Italy
10.1 Clinical and Pathophysiological Background
Twin pregnancies represent 1.2 % of spontaneously conceived pregnancies. Twins can either be dizygotic, after fertilization of two ova, or monozygotic, arising from the fertilization of a single ovum [1]. Only monozygotic (identical twin) can be monochorionic. The development of a monochorionic multiple gestation pregnancy is based on the day of embryonic splitting. If the embryo splits before day 3 after the fertilization, then two independent fetuses with separate placentas (dichorionic) will result. However, splitting after day 4 will result in a monochorionic diamniotic gestation. Division after day 8 results in a monochorionic monoamniotic gestation [1]. Owing to vascular connections within a single placenta, monochorionic gestations present distinctive prenatal management challenges, because an unbalanced hemodynamic exchange between fetuses could arise. This condition is called “twin to twin transfusion syndrome” (TTTS), and it is a serious progressive fetal pathology that occurs in 10–20 % of monochorionic diamniotic twin pregnancies [2]. In TTTS, disproportionate intertwin transfusion, via placental anastomoses, causes circulatory depletion in one twin (donor) and overload in the other (recipient) [2]. These anastomoses can be artery to artery (AA), vein to vein (VV), or artery to vein (AV) [3].
In TTTS, the most important and dangerous anastomosis is the AV shunt. AA and VV connections are in fact protective and can compensate for the AV shunt. The number and type of these connections determine whether the blood flow is balanced or unbalanced [4]. This instability may trigger further systemic changes that result in multiple organ injury, including the brain, in both twins during the second and third trimesters. This is one of the reasons because monochorionic twins are at an increased risk for complications compared to dichorionic twins. Consequently it is important to determine chorionicity (i.e., placenta number) in the ultrasonography of the first trimester (high accuracy for chorionicity already at 7–9 weeks) and then monitor the fetuses, with some ultrasound parameters that can be assessed for risk profiling for the development complications [5]. After the initial scan confirming presence of a monochorionic twin pair, continued ultrasound screening every 2 weeks starting at 16 weeks of gestation is recommended to allow for the early detection of twin–twin transfusion syndrome, selective fetal growth restriction, and twin anemia–polycythemia sequence (some other pathological conditions that can occur in monochorionic diamniotic pregnancy). Ultrasound surveillance should include assessment of amniotic fluid in each sac and determination of urine within both fetal bladders. The use of MRI is not yet included in the standard protocol for the surveillance of the monochorionic diamniotic pregnancies.
TTTS typically presents between 16 weeks and 26 weeks of gestation. If the disease is left untreated, the prognosis is poor, and overall mortality approaches 80–100 %. Fetoscopic laser coagulation (FLC) is the optimal treatment in TTTS [6], because it closes the pathological placental anastomoses. The reported survival of at least one twin is around 80 %, and 50 % of pregnancies treated by FLC result in the survival of both twins. However, 3–16 % of survivors are exposed to subsequent severe cerebral lesions on cranial ultrasonography [7], and 6–25 % suffer from neurodevelopmental impairment, either as a result of the disease itself or of the treatment or of preterm birth [8].
TTTS has variable clinical presentations, usually characterized as mild, moderate, or severe following the Quintero staging system, based on ultrasound and Doppler findings [9]. The severity of TTTS usually differs with the gestational age at which it presents, with earlier presentations tending to have more severe disease. Morbidity in this syndrome is a direct result of the pathophysiology. The donor suffers from hypovolemia and hypoxia, developing growth restriction and hypoplastic lungs (Fig. 10.1). The recipient experiences hypervolemia and can eventually develop hypertension and heart failure. Cardiac disease in the recipient is thought to be a major cause of death for both twins: with progressing cardiac dysfunction, the recipient develops hydrops and dies, precipitating hypotension in the donor [3]. This can lead to brain ischemia or death in the donor. The reverse occurs as well: if the donor should die, the recipient can develop hypotension resulting from the open placental anastomoses. In addition laser may worsen or cause brain lesions because of the abrupt hemodynamic changes imposed on the fetuses during the coagulation process or because of incidental bleeding [7]. Finally, it is important to underline how the death of one twin may occur unexpectantly in any moment, even in the absence of a TTTS syndrome. Co-twin death is a risk factor for brain ischemic injury in the survivor, due to the rapid blood shunting driven by gradient pressure drop towards the dead fetus.
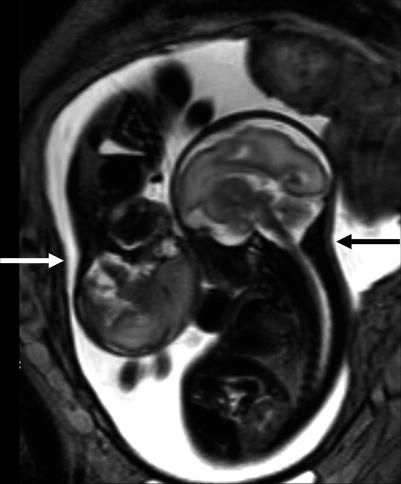

Fig. 10.1
Growth disproportion between donor (white arrow) and recipient (black arrow) is well documented on fetal MRI at 28 GW
10.2 Fetal and Postnatal MRI
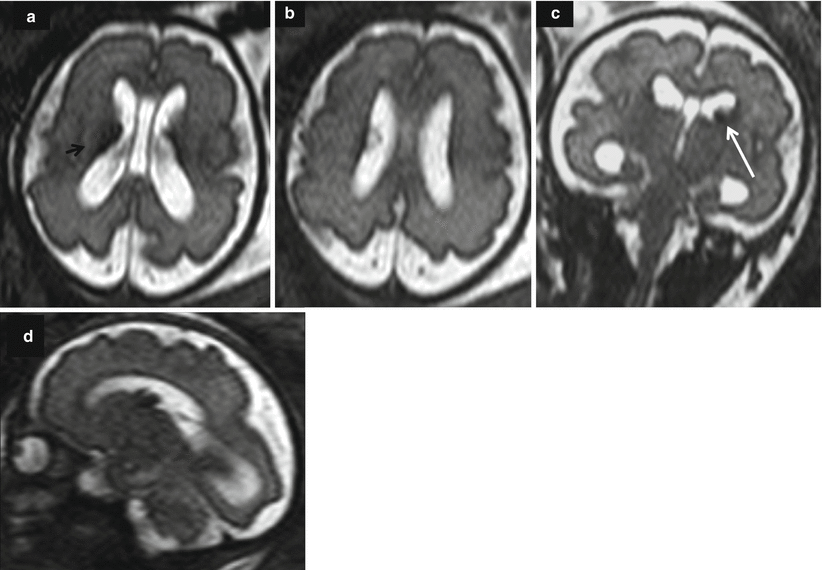
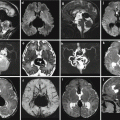
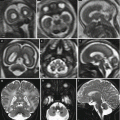
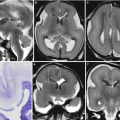
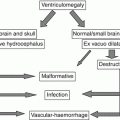
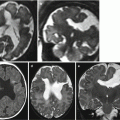
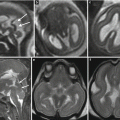
Fetal ultrasound is the method usually used in the surveillance of TTTS syndrome before and after the fetoscopic laser coagulation treatment, but it is relatively insensitive for evaluating parenchymal disease and gyration disorders [10]. MRI allows greater differentiation between gray and white matter and, with the use of DWI sequence, permits to detect early brain lesion after FLC treatment [11, 12]. It also helps to solve doubtful findings in fetal sonography. MRI acquisition is however challenging, because the median age in which the exam is performed is around 23 weeks [personal data], a period affected by great fetal movements; brain diameters are small and image spatial resolution limited. Despite these limits, MRI is superior to cranial ultrasound for detecting brain injury [10]. The major findings in TTTS are destructive lesions on ischemic–hemorrhagic basis such as periventricular leukomalacia, basal ganglia damage, germinal matrix, and intraventricular hemorrhage (Fig. 10.2) and cerebellar injury (Fig. 10.3), porencephaly, and hydranencephaly (Fig. 10.4). When such insult occurs early in gestation, it can lead to malformation of cortical development such as polymicrogyria (Fig. 10.5) or heterotopias (Fig. 10.6). The lesion type varies therefore on the basis of the timing and mechanism of injury [7].