Fig. 2.1
Cervix measurement
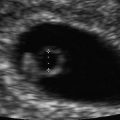
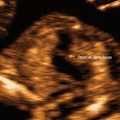
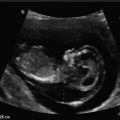
The baseline evaluation of the uterine body traditionally includes several features, comprising the overall size in standard dimensions of the uterus (length, height, width), position, the consistency of the endometrium, as well as any defects of the uterus or other pathology. The uterus is typically measured in the mid-sagittal plane for the longitudinal length and the height, measured in the anterior–posterior (AP) diameter (Fig. 2.2a). The length extends from the end of the cervix (external cervical os) to the top of the fundus. The transverse measurement of the uterus is also measured in the mid-corpus (see Fig. 2.2b). Additionally, the endovaginal ultrasound probe can be used as an extension of a pelvic examination to assess cornual tenderness, as well as a sliding organ sign, to show the movement of the ovaries in relation to the uterus, to establish fixed areas or adhesive disease [7]. Therefore, it is not only the images captured during the ultrasound, but much more information that can be gathered during the process of active sonography.
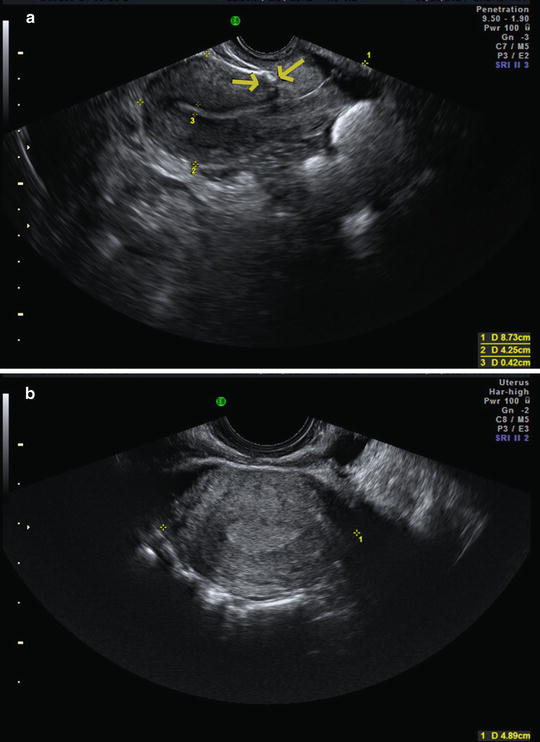

Fig. 2.2
Uterus measurements. (a) Longitudinal (1) and anterior-posterior (2) measurements for length and height. (b) Transverse view, 1 = width. The endometrium is thickened, consistent with the luteal phase. The arrows indicate the presence of a C-section scar
The size of the uterus corresponds well to the overall estrogenation of the body, with lower and normal/elevated estrogen values correlating with smaller and larger uterine size, respectively. The assumption that smaller uteri may be associated with adverse pregnancy outcomes is erroneous; in fact, larger uteri were more highly associated with ectopic pregnancies in IVF/ICSI cycles [8]. The position of the uterus is also routinely recorded as a dynamic measurement. This information is important for procedures such as embryo transfer, which is subsequently discussed; furthermore, if the uterus is noted to remain motionless on serial exams, the concern is raised for adhesive disease or an entrapped uterus [9].
The uterine evaluation may reveal factors that contribute to infertility or result in early pregnancy loss. Common uterine abnormalities include polyps, fibroids, intrauterine adhesions, cesarean section scars, and congenital uterine anomalies. Submucosal fibroids (Fig. 2.3a) may affect early reproductive outcomes, by impairing blood flow to the endometrium/myometrium, resulting in failed implantation and pregnancy loss. Surgical correction of these defects has been found to improve pregnancy outcomes. In contrast, intramural fibroids (see Fig. 2.3b) may also increase pregnancy loss, but surgical correction does not reduce the loss rate [10]. Some fibroids may grow large enough to impact the tubal ostia, making the passage of gametes into and out of the fallopian tube more difficult [11].


Fig. 2.3
Fibroids. (a) Intramural. (b) Subserosal
The endometrium is known to be a dynamic endocrine organ, which prepares itself and receives the developing embryo. Therefore, the endometrium is an important focus of the evaluation and treatment of the infertile patient. The overall thickness of the endometrium, when measured across the AP diameter, is correlated with the overall estrogenization of the pelvic organs. The endometrial lining is expected to be very thin during the initial part of the menstrual cycle, when estrogen levels are at a nadir, and is noted to increase around the time of ovulation and into the luteal phase, when the lining thickens in preparation of implantation (see Fig. 2.2b). Pathologies of the endometrium are known to affect reproductive outcomes, and are evaluated during the baseline examination. Intracavitary adhesions may be noted by an irregular or thin endometrium. Conversely, the presence of a thickened endometrial lining, on baseline ultrasound, may indicate a polyp or other defect is present within the endometrial cavity, which may need further evaluation to understand its impact on fertility. The thickness of the endometrium can be an indirect indicator of anovulation and possibly hyperplasia.
The ultrasound echo pattern of the endometrium is typically noted and followed during a treatment cycle. In the follicular phase, the endometrium grows in thickness and has a trilaminar appearance (Fig. 2.4). After ovulation, the endometrium becomes uniformly hyperechoic in this luteal phase portion of the menstrual cycle. These patterns have not correlated with pregnancy outcomes but this is often used as a part of the clinical assessment.


Fig. 2.4
Proliferative endometrium with trilaminar appearance
The presence of endometrial glands and stroma located within the myometrium is termed, adenomyosis. Adenomyosis is known to be clinically associated with dysmenorrhea, abnormal uterine bleeding and pelvic pain [12]. Adenomyosis is traditionally diagnosed histologically; however, several ultrasonographic features are thought to indicate the presence of adenomyosis (Fig. 2.5). These include cystic areas in the myometrium as well as increased vascularity along the periphery of the uterine body [13]. These findings have been described as “venetian blinds” secondary to the shadowing produced by these defects on structures further from the ultrasound probe [14]. In addition, one can demonstrate asymmetry of the anterior and posterior aspects (in relation to the endometrium) of the myometrium. New information suggests that the presence of adenomyosis decreases reproductive outcomes after infertility treatment such as in vitro fertilization (IVF). Therefore, some sources are recommending sonographic screening for adenomyosis in the subfertile population [15].

Fig. 2.5
Adenomyosis
Ultrasound is used to rule out any suspicion of congenital abnormality of the uterus. Congenital uterine anomalies require 3D ultrasound or MRI to make the diagnosis, since the coronal surface of the uterus must be evaluated along with the endometrial cavity to distinguish between an arcuate uterus, a septate or subseptate uterus (Fig. 2.6), and a bicornuate uterus [16]. The luteal phase is the best time to perform a 3D ultrasound to assess for a congenital uterine anomaly since the endometrium will be thickened and hyperechoic and thus will act as its own contrast material [16].


Fig. 2.6
Congenital uterine anomaly: subseptate uterus with a pregnancy in one horn
Ovaries
As part of the baseline ultrasound evaluation, both ovaries are identified, measured in three dimensions, their position described (especially if located high out of the pelvis or posterior to the uterus) and the number of follicles (2–9 mm) in each ovary is counted (Fig. 2.7a, b). Any ovarian cysts or masses are described and further evaluation of these cysts/masses is performed with color or power Doppler.


Fig. 2.7
Ovaries. (a) Longitudinal view in parallel with iliac vessels. 1 = length; 2 = height. (b) Transverse view with iliac vessel in transverse view (circle) with ovary width (3)
The iliac vessels are used as a guide to find the ovaries and to determine the measurements. The length of the ovary is parallel to the length of the iliac vessel and the height is perpendicular to these measurements (see Fig. 2.7). Next, an orthogonal view of the same organ is performed, resulting in a transverse view of the iliac vessel (circle) with the ovary above it. The width of the ovary is measured in this transverse view. The ovarian volume can be determined using a modified ellipsoid formula or a 3D volume. The ovarian size can be diminished by hormonal contraceptives, smoking, menopause (including premature menopause), radiation, among other disorders. A large ovarian volume (>10 cm3) is one of the measurements associated with a polycystic ovary appearance (Fig. 2.8) [6]. As expected, ovaries with cysts or masses will measure larger than normal.


Fig. 2.8
A polycystic ovary (PCO). Several follicles are demonstrated in the organ. Periphery and the total volume of the ovary is increased above 10 cc
In the infertility population like all reproductive age women, ultrasound is most likely to identify benign ovarian pathology when an ovarian mass is present. Physiologic or simple cysts may be commonly visualized as follicular cysts, which are anechoic with no internal debris and usually round or potentially collapsed after ovulation (Fig. 2.9). These are thin-walled with posterior enhancement, and no internal color flow with Doppler ultrasound [17].


Fig. 2.9
Simple ovarian cyst. No Doppler flow within the cyst
Other commonly visualized cysts include hemorrhagic corpus luteum cysts, endometriomas, and mature teratomas. Hemorrhagic corpus luteum cysts can have several appearances from an initial simple cyst when the blood is still liquid to more complex cystic masses, as the blood organizes into clots, which gives the appearance of a reticular pattern of internal echoes (a lacy appearance, generally due to fibrin strands) and/or, lastly, a combination appearance (cystic and solid), with solid-appearing area with concave margins, no internal flow on color Doppler ultrasound, and fluid (Fig. 2.10a). Usually the ovarian wall around the cyst has circumferential Doppler flow (see Fig. 2.10b) [17].


Fig. 2.10
(a) Hemorrhagic corpus luteum with solid and cystic components. (b) Hemorrhagic corpus luteum cysts. Internal echoes can be seen (organized clots, with reticular pattern due to fibrin strands). No internal flow is seen on color Doppler US but circumferential flow is clearly demonstrated (“ring of fire”)
The typical endometriomas have internal homogeneous low-level echoes, sometimes described as a “ground glass” appearance, and have no internal color Doppler flow, wall nodules, or other neoplastic features. In such masses, the additional features of multilocularity and/or tiny echogenic wall foci may occur (Fig. 2.11a, b) [17]. Small endometriomas often do not need intervention and have not been found to affect reproductive outcomes [18–20].


Fig. 2.11
(a) Endometrioma. (b) Endometrioma with atypical findings. The image shows the low-level echoes consistent with an endometrioma. The atypical features include the irregular borders and the hyperechoic small nodules
“Dermoids” as they are commonly known are mature cystic teratomas of the ovary, consisting of sebaceous material, hair, and teeth. The ultrasound appearances of dermoids consist of focal or diffuse hyperechoic components, hyperechoic lines and dots, and area of acoustic shadowing, with no internal flow with color Doppler ultrasound (Fig. 2.12) [17]. Some have a nodule with shadowing called Rotkitansky’s nodule. Color and/or power Doppler should be used to assess adnexal masses. No Doppler flow should be going into the dermoid or Rotkitansky’s nodule. All abnormal findings need to be monitored with serial ultrasounds [17].


Fig. 2.12
Dermoid or mature teratoma. Courtesy of Leeber Cohen, MD
Other presentations to note include that of premature ovarian insufficiency patient, in whom the ovaries will be much smaller, consistent with menopausal patients and few or no antral follicles will be visualized. This finding helps establish the diagnosis, in a patient presenting with unexplained amenorrhea, and may help with fertility counseling.
Adnexa
Another critical component of the baseline ultrasound is to evaluate for any adnexal pathology. The most commonly encountered tubal findings are hydrosalpinx (Fig. 2.13a) and paratubal cysts or cysts of Morgagni. Both of these findings can be confused with a dominant follicle, instead of a diseased tube, and it is important that the provider performing the ultrasound clearly assesses the location of the pathology (in, versus adjacent to, the ovary) and view the pathology in three dimensions, to ensure that most information is gathered from the study (see Fig. 2.13b).


Fig. 2.13
Hydosalpinx. (a) Large hydrosalpinx. (b) Hydosalpinx en face can be confused with a blood vessel
Miscellaneous
A variety of other findings are noted on the baseline ultrasound of the patient presenting with infertility. These may include free fluid around the ovaries or in the posterior cul-de-sac, as well as abnormalities in the bowel or surrounding structures. All abnormalities should be noted in the report.
Saline Infusion Sonohysterogram
During the initial workup of the infertile patient, in addition to the baseline ultrasound, one needs to perform an evaluation of the uterine cavity and an assessment of tubal patency. This information is critical in the decision of need and type of treatment offered to the patient.
The uterine cavity is best evaluated as soon as possible after the menses is completed and before ovulation (cycle days 6–12). This evaluation has traditionally been a radiographic procedure, called a hysterosalpingogram (HSG) in which contrast dye is injected through a cannula into the uterine cavity under fluoroscopic guidance and x-rays are taken. Ultrasound can be used with saline infusion to perform a similar procedure, called a saline infusion sonohysterogram (SIS). This SIS procedure is reported in several studies to be superior to the HSG in evaluating the uterine cavity [21]. Although similar information is gathered from an HSG, there is no radiation exposure during an SIS. Being able to perform and interpret the ultrasound exam in real time is a benefit of the SIS, and, unlike an HSG, this test may also reveal the diagnosis resulting in the abnormal filling defect (i.e., polyp, fibroid, adhesions). An SIS procedure is best performed in the early follicular phase after the cessation of menses, and after a baseline ultrasound has been performed.
During an SIS procedure, a patient is placed in lithotomy position, vagina and cervix are prepped, and then, typically, a small balloon or acorn catheter is placed into the cervix or endometrial cavity. If a balloon is used, this balloon is then inflated to create a seal, preventing liquid from escaping the uterine cavity through the cervix. Sterile saline is then injected and the uterine cavity is thoroughly examined in multiple planes, to detect any filling defects (Fig. 2.14a, b), such as polyps (Fig. 2.15) or submucosal fibroid (Fig. 2.16) [22]. SIS used with color or power Doppler cannot only identify a filling defect (as noted by HSG), but also can identify the nature of the defect (i.e., polyp, fibroid, adhesion, etc.). Other pathology, which can be detected, includes endometrial adhesions, also known as Asherman syndrome (Fig. 2.17). In a population of subfertile women, SIS has been shown to be a highly sensitive tool and comparable to the gold standard tool, hysteroscopy in the detection of intrauterine abnormalities [23]. The evaluation of the uterine cavity has traditionally been performed utilizing 2D ultrasonography, with the operator examining and sweeping through the cavity in sagittal and coronal planes, to evaluate the entire uterine cavity. Evidence, however, is now mounting regarding the use and possible superiority of 3D ultrasound for the assessment of the uterine cavity, which is discussed later in the chapter.





Fig. 2.14
Saline infusion sonohysterogram (SIS). (a) Sagittal view showing the longitudinal axis of the uterus with fluid, which appears black in the uterine cavity. No intrauterine filling defects. (b) Transverse view of the uterus with fluid in the uterine cavity at the mid-uterine level

Fig. 2.15
(a) SIS of the uterus revealing a sessile polyp which measured at approximately 1 cm. (b) 3D SIS with polyp

Fig. 2.16
SIS with submucosal fibroid

Fig. 2.17
SIS with intrauterine adhesions (Asherman syndrome)
If a uterine anomaly is suspected, three-dimensional sonography (3D ultrasound) is often required to confirm the diagnosis. 3D ultrasound is also helpful in identifying the location of any intrauterine pathologies or the placement of an intrauterine device.
A more controversial topic is the use of ultrasound with agitated saline, in a saline sonosalpingogram (SSS) to study tubal patency. This new term differentiates the assessment of the cavity (SIS) from the tubes (SSS). SSS may require a different skill levels to perform this examination.
Either preceding the evaluation of the uterine cavity or after, the fallopian tubes can be assessed. This is completed by the injection of agitated saline into the uterine cavity. Saline can be agitated either manually or by commercially available product. A new FDA-approved device, Femvue, can be used to detect tubal patency, through the mechanized installation of saline with bubbles into the cavity and fallopian tubes. Utilizing a transverse view of the uterine fundus near the cornua, agitated saline can be visualized with air bubbles traversing the proximal fallopian tubes. More specifically, the cornual portion of the uterus can be identifiable as a “lemon-appearing” transverse view of the uterus. It is often apparent to see a pencil thin line going from the endometrium into the proximal tube; however, if this is not observed, then the passage of the echogenic bubbles can be visualized traversing the cornua.
After both sides are examined, a thorough inspection of the pelvis ensues, either to detect a hydrosalpinx or to detect free fluid around one or both ovaries, or in the cul-de-sac. Of note, if agitated saline is not clearly visualized extruding through the uterine cornua, but free fluid was noted, at least unilateral tubal patency has been confirmed. Once the uterine cavity and fallopian tubes are assessed, the balloon is deflated and procedure terminated [22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree