Fig. 4.1
Algorithm for ultrasound assessment of undifferentiated shock, modified from the RUSH protocol. The algorithm begins with critical care echocardiography, with subsequent focused exams as described in the chapter text
Following the initial echocardiogram, the clinician should prioritize the following studies based upon clinical suspicion. If the clinical picture is unclear, we suggest cardiac echo, followed by IVC ultrasound, then abdominal, including the aorta, followed by lung pleura, and finally a lower extremity venous study. This approach allows the clinician to minimize probe and location changes.
Goal-Directed Echocardiography
A rapid four-view echocardiogram utilizing parasternal long and short axis views, an apical four-chamber view, and a subcostal view should be performed. This will give an assessment of overall left ventricle (LV) function, size and function of the right ventricle (RV), and the presence of pericardial effusion and possible tamponade. See Chaps. 5, 6, and 7 on critical care echocardiography for complete details.
IVC Ultrasound
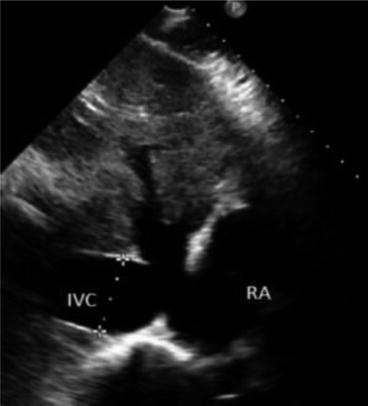
From the subxiphoid position used for echocardiography slide the probe to 1–2 cm to the patient’s right, the inferior vena cava (IVC) is examined by fanning and sliding the probe to assess the vessel’s diameter and collapsibility. See below for discussion of volume assessment.
Abdominal Exam
The four views of the FAST exam should be obtained: right upper quadrant (RUQ), subcostal, left upper quadrant (LUQ), and suprapubic to rapidly assess the presence of free fluid in the peritoneal cavity. Please refer to Chap. 12 for full details.
Aorta
In brief, the probe is placed in the midline, the aorta identified, and then tracked from a cephalad position in the abdomen to its bifurcation into the iliac arteries. Both transverse and longitudinal views of the aorta are obtained to assess for aneurysm. See Chap. 12 for the details of this exam.
Pleural Ultrasound
Three to four sites are examined on each hemithorax to confirm presence or absence of lung sliding as well as identifying any clinically significant pleural effusion. Lack of lung sliding may indicate pneumothorax, while a large pleural effusion may represent empyema or hemothorax. Additional images and details on pleural ultrasound can be found in Chap. 10.
Assessment of Venous Thrombosis
Switching to a high frequency probe, identify the femoral vein and track the deep veins distally to the level of the popliteal vein. Assess for echogenic material within the vessel, lack of compressibility, or abnormal Doppler flow to diagnose deep vein thrombosis (DVT). Lack of vessel compressibility is the most significant finding suggestive of deep venous thrombosis. Full details and images of venous ultrasound can be found in Chap. 13.
Evidence-Based Review
Rapid diagnostic assessment and implementation of appropriate treatment in a critically ill patient can be lifesaving. In the emergency room setting, the early use of bedside ultrasound has been shown to be superior to delayed use of ultrasound in formulating a clinically meaningful differential diagnoses. In patients with undifferentiated, nontraumatic, shock, Jones et al. compared the ability to correctly diagnose shock between those who were immediately evaluated with bedside ultrasound versus implementation of 15–30 min of standard care followed by a delayed ultrasound. Physicians in the early ultrasound group had a more focused differential diagnosis and were able to correctly identify shock etiology in 80 % of cases 15 min into the resuscitation, while the traditional management algorithm correctly identified shock etiology only 50 % of the time [3]. Despite this supportive data, and the intuitive benefit of the use of bedside ultrasound in the assessment of undifferentiated shock, there is limited prospective data validating the use of ultrasound-based algorithms to diagnose and manage shock.
Ultrasound offers the advantages of rapid, noninvasive, point of care use, which produces data that is often superior to the traditional physical exam. Brennan et al. substantiated this assertion in a study that showed medical students with limited didactic and ultrasound training were more accurately able to estimate right atrium (RA) pressures with ultrasound than with traditional jugular venous pressure measurements (90 vs. 63 %) [4]. The superiority of ultrasound over the physical exam was further demonstrated by Kobal et al. who compared medical students using handheld ultrasound with the physical exams of board certified cardiologists in diagnosing common cardiac conditions. Following 18 h of ultrasound training, the medical students significantly outperformed the cardiologists in all measured categories [5]. Clearly, the use of ultrasound by physicians to diagnose and direct management patient care at the bedside is rapidly expanding. Further elaboration on ultrasound training programs and standards can be found in Chap. 2.
Volume Status and Fluid Responsiveness
Introduction
Physicians frequently face the clinical challenge of accurately assessing a critically ill patient’s volume status; moreover, predicting which patients will be responsive to fluids is an elusive task. Clinical parameters such as vital signs and the physical exam have been shown to be imprecise, are variably interpreted amongst providers, and are therefore unable to accurately predict the hemodynamic status of critically ill patients [6, 7]. The traditional gold standard of hemodynamic monitoring, pulmonary artery catheterization, is invasive, labor-intensive, and of questionable benefit when used routinely in intensive care unit (ICU) patients [8]. With pulmonary artery catheterization now performed infrequently during the management of the critically ill, clinicians are in need of a dependable, noninvasive modality to estimate volume status and fluid responsiveness.
Central venous pressure (CVP) is the most commonly used static measure of preload and is currently recommended by internationally endorsed guidelines for treating patients with septic shock [9]. While IVC collapsibility has been shown to correlate with low CVP [10], static measures such as CVP are poor surrogates for cardiac preload and are unable to predict which patients will respond to fluid loading [11]. A meta-analysis by Marik and Cavallazzi illustrated that CVP is unable to predict fluid responsiveness (FR) and concluded that “there are no data to support the widespread practice of using CVP to guide fluid therapy” [12]. Furthermore, the results of the Protocolized Care for Early Septic Shock (ProCESS) trial question the utility of routinely placing central lines in patients with septic shock who do not require vasopressors [13]. These findings have led a number of physicians to look for alternatives to CVP, and instead use dynamic and noninvasive modalities to direct fluid resuscitation. Ultrasound measurement of the IVC diameter and caval index (the relative change in diameter observed during respiration) has been proposed as a noninvasive means of determining volume status and identifying those patients whose cardiac output may be improved by further volume administration.
Technical and Patient Considerations
In general, when assessing volume status and responsiveness the best images are acquired with the patient in the supine position. However, there are a number of clinical circumstances where laying a patient flat will not be possible; for example, a patient with severe orthopnea is unlikely to tolerate supine positioning. In these circumstances, keeping the patient semi-recumbent at a 30–45° angle is appropriate. Be aware that semi-recumbent positioning will modify the IVC diameter; however, the magnitude of its effect is not clearly established. Obese patients and abdominal bowel gas pose challenges to image acquisition. Properly adjusting depth and gain can help to maximize image quality. Applying gentle pressure with the probe to the abdomen, while resting the hand holding the probe on the abdominal wall, will help clear bowel gas from the study image. Constant contact with the probe hand is important, since proprioception will provide feedback and prevent the application of undue pressure to the patient’s abdomen.
Image Acquisition and Interpretation
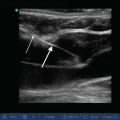
For proper IVC image acquisition select the low frequency (1–5 MHz) phased array probe, as this will provide optimal abdominal penetration and visualization of deep structures. With the patient in the supine position, rest the hand holding the probe on the patient’s epigastric area (subxiphoid view) with the probe marker directed cranially and the probe angled about 45° off the skin (Fig. 4.2). Apply gradual pressure with the lateral aspect of the smallest finger in direct contact with the patient’s abdomen, then slide the probe a few centimeters to the patient’s right. As the probe moves off the midline, the long-axis IVC will come into view. The IVC can be identified as it transverses the liver, where the hepatic veins feed into it, and it can be followed cranially to where it terminates in the right atrium. Some general characteristics of the IVC can be observed that may immediately suggest a given physiologic state. For example, if the clinician observes an IVC that completely collapses during respiration, this strongly suggests volume depletion and does not necessarily require direct measurement [14]. A more formal approach to quantifying the collapsibility of the IVC, known as the caval index, will be outlined below.
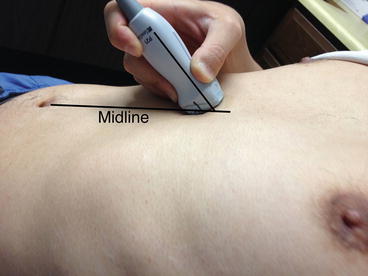

Fig. 4.2
Transducer placement to examine the inferior vena cava (IVC). Note the transducer is slightly shifted to patient’s right from midline. Also note it is tilted almost 45° to cephalad (Image by Dr. Taro Minami)
Occasionally the patient’s body habitus or abdominal gas may make it impossible to adequately visualize the IVC from the subxiphoid approach. In this case, the examiner should scan from the lateral chest/abdominal wall, using the same technique as in the FAST exam or when performing an ultrasound of the right hemidiaphragm. This alternative approach will often provide a non-obscured view of IVC (see Chap. 12 for further imaging details).
Basic Competencies
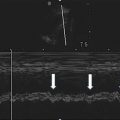
To obtain IVC measurements ensure that the center of the IVC is being imaged in the long-axis view. It is important to obtain images of the IVC in the center of its lumen as lateral displacement of the probe in either direction will affect the measurement of the vessel’s diameter. Obtain IVC diameter measurements at maximal inspiration and maximal expiration 3 cm caudal from the right atrial boarder or just proximal to the junction of the hepatic veins and the IVC. Both locations reliably produce consistent measurements (Fig. 4.3). Optimal measurements are obtained by recording a video clip of the IVC in B-mode and performing frame-by-frame analysis with calipers to record maximal and minimal caval diameters. Alternatively using M-mode to obtain a time-motion record of the IVC diameter is also an effective strategy (Fig. 4.4).